

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM506 3-({4-[bis(4-hydroxy-2-oxo-2H-chromen-3-yl)methyl]phenyl}(4-hydroxy-2-oxo-2H-chromen-3-yl)methyl)-4-hydroxy-2H-chromen-2-one::CHEMBL293914::Cancer Chemotherapy National Service Center (CCNSC) compound 158393::NSC 158393
SMILES: Oc1c(C(c2ccc(cc2)C(c2c(O)c3ccccc3oc2=O)c2c(O)c3ccccc3oc2=O)c2c(O)c3ccccc3oc2=O)c(=O)oc2ccccc12
InChI Key: InChIKey=BXKKDJUEVYKLOT-UHFFFAOYSA-N
Data: 12 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIV-1 Protease (Human immunodeficiency virus type 1) | BDBM506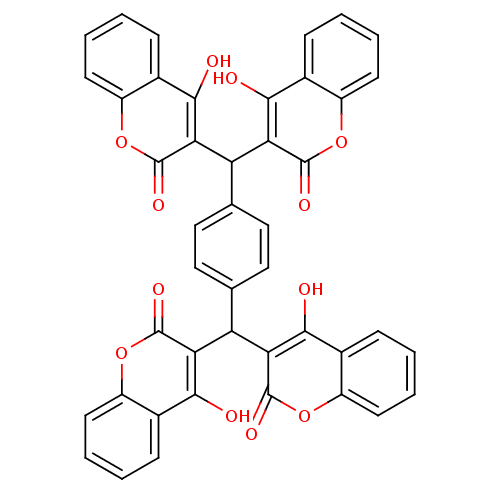 (3-({4-[bis(4-hydroxy-2-oxo-2H-chromen-3-yl)methyl]...) | PDB GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | 6.5 | 37 |
National Institutes of Health | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 39: 2047-54 (1996) Article DOI: 10.1021/jm950874+ BindingDB Entry DOI: 10.7270/Q2S46Q4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 integrase (Human immunodeficiency virus 1) | BDBM506 (3-({4-[bis(4-hydroxy-2-oxo-2H-chromen-3-yl)methyl]...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 integrase | J Med Chem 39: 2472-81 (1996) Article DOI: 10.1021/jm960074e BindingDB Entry DOI: 10.7270/Q2DR2W5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 integrase (Human immunodeficiency virus 1) | BDBM506 (3-({4-[bis(4-hydroxy-2-oxo-2H-chromen-3-yl)methyl]...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibitory concentration against purified recombinant integrase by strand transfer in vitro assay | J Med Chem 40: 930-6 (1997) Article DOI: 10.1021/jm960754h BindingDB Entry DOI: 10.7270/Q24X56VR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 integrase (Human immunodeficiency virus 1) | BDBM506 (3-({4-[bis(4-hydroxy-2-oxo-2H-chromen-3-yl)methyl]...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibitory concentration against purified recombinant integrase by 3'-processing in vitro assay | J Med Chem 40: 930-6 (1997) Article DOI: 10.1021/jm960754h BindingDB Entry DOI: 10.7270/Q24X56VR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 integrase (Human immunodeficiency virus 1) | BDBM506 (3-({4-[bis(4-hydroxy-2-oxo-2H-chromen-3-yl)methyl]...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Integration of DNA by HIV -1 integrase | J Med Chem 40: 242-9 (1997) Article DOI: 10.1021/jm960450v BindingDB Entry DOI: 10.7270/Q2DN444V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase I (Topo I) (Homo sapiens (Human)) | BDBM506 (3-({4-[bis(4-hydroxy-2-oxo-2H-chromen-3-yl)methyl]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibitory activity against topoisomerase I. | J Med Chem 39: 2472-81 (1996) Article DOI: 10.1021/jm960074e BindingDB Entry DOI: 10.7270/Q2DR2W5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD(P)H dehydrogenase [quinone] 1 (Homo sapiens (Human)) | BDBM506 (3-({4-[bis(4-hydroxy-2-oxo-2H-chromen-3-yl)methyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human recombinant NQO1 in presence of BSA | J Med Chem 50: 6316-25 (2007) Article DOI: 10.1021/jm070472p BindingDB Entry DOI: 10.7270/Q2DV1JM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD(P)H dehydrogenase [quinone] 1 (Homo sapiens (Human)) | BDBM506 (3-({4-[bis(4-hydroxy-2-oxo-2H-chromen-3-yl)methyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human recombinant NQO1 | J Med Chem 50: 6316-25 (2007) Article DOI: 10.1021/jm070472p BindingDB Entry DOI: 10.7270/Q2DV1JM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 protease (Human immunodeficiency virus type 1) | BDBM506 (3-({4-[bis(4-hydroxy-2-oxo-2H-chromen-3-yl)methyl]...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Protease. | J Med Chem 39: 2472-81 (1996) Article DOI: 10.1021/jm960074e BindingDB Entry DOI: 10.7270/Q2DR2W5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 2 integrase (Human immunodeficiency virus 2) | BDBM506 (3-({4-[bis(4-hydroxy-2-oxo-2H-chromen-3-yl)methyl]...) | UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibitory activity against HIV-2 Integrase was measured using 3'-processing (3'-proc) assay. | J Med Chem 39: 2472-81 (1996) Article DOI: 10.1021/jm960074e BindingDB Entry DOI: 10.7270/Q2DR2W5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 integrase (Human immunodeficiency virus 1) | BDBM506 (3-({4-[bis(4-hydroxy-2-oxo-2H-chromen-3-yl)methyl]...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 integrase | J Med Chem 39: 2472-81 (1996) Article DOI: 10.1021/jm960074e BindingDB Entry DOI: 10.7270/Q2DR2W5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 integrase (Human immunodeficiency virus 1) | BDBM506 (3-({4-[bis(4-hydroxy-2-oxo-2H-chromen-3-yl)methyl]...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibitory activity against 3'-processing of DNA by HIV-1 integrase | J Med Chem 40: 242-9 (1997) Article DOI: 10.1021/jm960450v BindingDB Entry DOI: 10.7270/Q2DN444V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||