

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: COc1ccc(cc1)C(=O)Nc1cccc(\C=C(/CCC(O)=O)c2nc3ccccc3s2)c1
InChI Key: InChIKey=VOYVZKMRCLUBFD-XDJHFCHBSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50625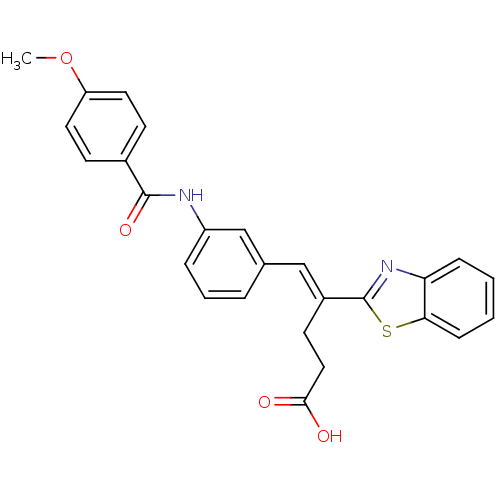 ((E)-4-(1,3-benzothiazol-2-yl)-5-[3-(p-anisoylamino...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 4.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Molecular Libraries Screening Center Curated by PubChem BioAssay | Assay Description NIH Molecular Libraries Screening Centers Network [MLSCN] Emory Chemical Biology Discovery Center in MLSCN Assay provider: Nikolovska-Coleska, Univer... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q23X8539 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent N-type calcium channel subunit alpha-1B (Homo sapiens (Human)) | BDBM50625 ((E)-4-(1,3-benzothiazol-2-yl)-5-[3-(p-anisoylamino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 4.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute Network... | PubChem Bioassay (2009) BindingDB Entry DOI: 10.7270/Q2PZ578V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Streptokinase A (Streptococcus pyogenes M1 GAS) | BDBM50625 ((E)-4-(1,3-benzothiazol-2-yl)-5-[3-(p-anisoylamino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a |
Broad Institute Curated by PubChem BioAssay | Assay Description Keywords: Group A streptococcus, GAS, streptokinase, expression, virulence, inhibition, dose response, EC50 Assay Overview: The goal of this assa... | PubChem Bioassay (2009) BindingDB Entry DOI: 10.7270/Q2736PBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M1 family aminopeptidase (Plasmodium falciparum (isolate FcB1 / Columbia)) | BDBM50625 ((E)-4-(1,3-benzothiazol-2-yl)-5-[3-(p-anisoylamino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 2.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SRMLSC Curated by PubChem BioAssay | Assay Description Southern Research Molecular Libraries Screening Center (SRMLSC) Southern Research Institute (Birmingham, Alabama) NIH Molecular Libraries Screening... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q27H1H0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||