

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CCn1cc(C(=O)Nc2ccc(Oc3ccnc4[nH]nc(N[C@H](C)CO)c34)c(F)c2)c(=O)c(c1)-c1ccc(F)cc1
InChI Key:
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM517045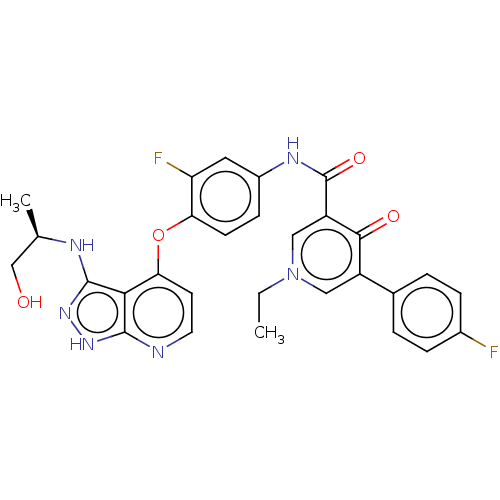 ((R)-1-ethyl-N-(3-fluoro- 4-((3-((1- hydroxypropan-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The affinity of compound binding to wild type and mutant human MET kinases is measured using Invitrogen's LanthaScreen™ Eu Kinase Binding technol... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BZ6962 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM517045 ((R)-1-ethyl-N-(3-fluoro- 4-((3-((1- hydroxypropan-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The affinity of compound binding to wild type and mutant human MET kinases is measured using Invitrogen's LanthaScreen™ Eu Kinase Binding technol... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BZ6962 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM517045 ((R)-1-ethyl-N-(3-fluoro- 4-((3-((1- hydroxypropan-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The affinity of compound binding to wild type and mutant human MET kinases is measured using Invitrogen's LanthaScreen™ Eu Kinase Binding technol... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BZ6962 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM517045 ((R)-1-ethyl-N-(3-fluoro- 4-((3-((1- hydroxypropan-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The affinity of compound binding to wild type and mutant human MET kinases is measured using Invitrogen's LanthaScreen™ Eu Kinase Binding technol... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BZ6962 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor [1-13,15-1390] (Homo sapiens (Human)) | BDBM517045 ((R)-1-ethyl-N-(3-fluoro- 4-((3-((1- hydroxypropan-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The affinity of compound binding to wild type and mutant human MET kinases is measured using Invitrogen's LanthaScreen™ Eu Kinase Binding technol... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BZ6962 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor [Y1230S] (Homo sapiens (Human)) | BDBM517045 ((R)-1-ethyl-N-(3-fluoro- 4-((3-((1- hydroxypropan-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The affinity of compound binding to wild type and mutant human MET kinases is measured using Invitrogen's LanthaScreen™ Eu Kinase Binding technol... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BZ6962 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor [D1228N] (Homo sapiens (Human)) | BDBM517045 ((R)-1-ethyl-N-(3-fluoro- 4-((3-((1- hydroxypropan-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The affinity of compound binding to wild type and mutant human MET kinases is measured using Invitrogen's LanthaScreen™ Eu Kinase Binding technol... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BZ6962 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor [F1200I] (Homo sapiens (Human)) | BDBM517045 ((R)-1-ethyl-N-(3-fluoro- 4-((3-((1- hydroxypropan-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The affinity of compound binding to wild type and mutant human MET kinases is measured using Invitrogen's LanthaScreen™ Eu Kinase Binding technol... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BZ6962 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor [L1195V] (Homo sapiens (Human)) | BDBM517045 ((R)-1-ethyl-N-(3-fluoro- 4-((3-((1- hydroxypropan-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The affinity of compound binding to wild type and mutant human MET kinases is measured using Invitrogen's LanthaScreen™ Eu Kinase Binding technol... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BZ6962 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor [Y1230C] (Homo sapiens (Human)) | BDBM517045 ((R)-1-ethyl-N-(3-fluoro- 4-((3-((1- hydroxypropan-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The affinity of compound binding to wild type and mutant human MET kinases is measured using Invitrogen's LanthaScreen™ Eu Kinase Binding technol... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BZ6962 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor [Y1230H] (Homo sapiens (Human)) | BDBM517045 ((R)-1-ethyl-N-(3-fluoro- 4-((3-((1- hydroxypropan-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The affinity of compound binding to wild type and mutant human MET kinases is measured using Invitrogen's LanthaScreen™ Eu Kinase Binding technol... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BZ6962 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor [D1228H] (Homo sapiens (Human)) | BDBM517045 ((R)-1-ethyl-N-(3-fluoro- 4-((3-((1- hydroxypropan-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The affinity of compound binding to wild type and mutant human MET kinases is measured using Invitrogen's LanthaScreen™ Eu Kinase Binding technol... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BZ6962 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||