

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: C[C@@H]1CN(CCN1)c1ccc(cn1)-c1n[nH]c2cnc(nc12)N1[C@@H](C)CCC[C@H]1C
InChI Key:
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Activin receptor type-1 [147-509] (Homo sapiens (Human)) | BDBM518135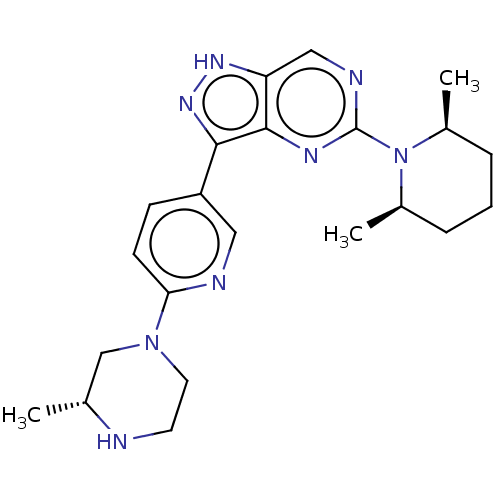 (US11111247, Example 1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description ALK2 (aa 147-end) was obtained from BPS biosciences. The enzymatic assays were conducted in white 384-well polystyrene plates in a final volume of 8 ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2571G50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 [147-509] (Homo sapiens (Human)) | BDBM518135 (US11111247, Example 1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description ALK2 (aa 147-end) was obtained from BPS biosciences. The enzymatic assays were conducted in white 384-well polystyrene plates in a final volume of 8 ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2571G50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 [147-509] (Homo sapiens (Human)) | BDBM518135 (US11111247, Example 1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description ALK2 (aa 147-end) was obtained from BPS biosciences. The enzymatic assays were conducted in white 384-well polystyrene plates in a final volume of 8 ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2571G50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM518135 (US11111247, Example 1) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 384 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00206 BindingDB Entry DOI: 10.7270/Q2R78K72 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 2 (Homo sapiens (Human)) | BDBM518135 (US11111247, Example 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Phosphorylated (P): The inhibitor potency of the exemplified compounds was determined in an enzyme discontinuous assay that measures peptide phosphor... | Citation and Details BindingDB Entry DOI: 10.7270/Q2571G50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM518135 (US11111247, Example 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description un-Phosphorylated (UP): The inhibitor potency of the exemplified compounds was determined in an enzyme discontinuous assay that measures peptide phos... | Citation and Details BindingDB Entry DOI: 10.7270/Q2571G50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM518135 (US11111247, Example 1) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00206 BindingDB Entry DOI: 10.7270/Q2R78K72 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM518135 (US11111247, Example 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description un-Phosphorylated (UP): The inhibitor potency of the exemplified compounds was determined in an enzyme discontinuous assay that measures peptide phos... | Citation and Details BindingDB Entry DOI: 10.7270/Q2571G50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||