

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CC(C)C[C@@H]1N2[C@H](O)CC[C@H](NC(=O)[C@H](CC#C)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)C(C)NC(=O)OCc3ccccc3)[C@@H](C)OC(=O)[C@@H](NC(=O)[C@H](Cc3ccccc3)N(C)C1=O)C(C)C)C2=O
InChI Key:
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutrophil elastase (Homo sapiens (Human)) | BDBM521758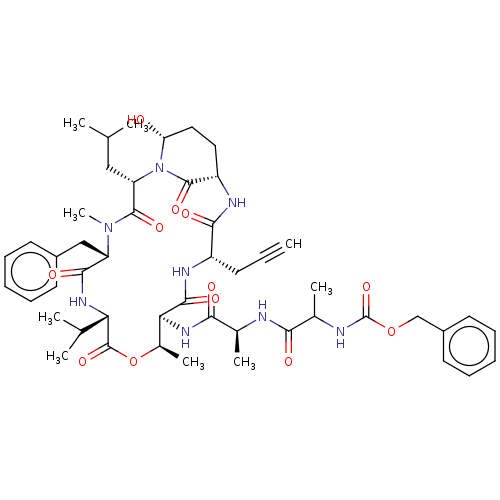 (US11149067, Compound Ahp18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA3 [111-436] (Human) | BDBM521758 (US11149067, Compound Ahp18) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | 1.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 [158-480] (Homo sapiens (Human)) | BDBM521758 (US11149067, Compound Ahp18) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||