

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM5414 benzyl(2-methoxyethyl)(2-{4-[3-(thiophen-3-yl)pyrazolo[1,5-a]pyrimidin-6-yl]phenoxy}ethyl)amine::pyrazolo[1,5-a]pyrimidine 4f
SMILES: COCCN(CCOc1ccc(cc1)-c1cnc2c(cnn2c1)-c1ccsc1)Cc1ccccc1
InChI Key: InChIKey=SYXUIDNWCZVPRH-UHFFFAOYSA-N
Data: 1 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM5414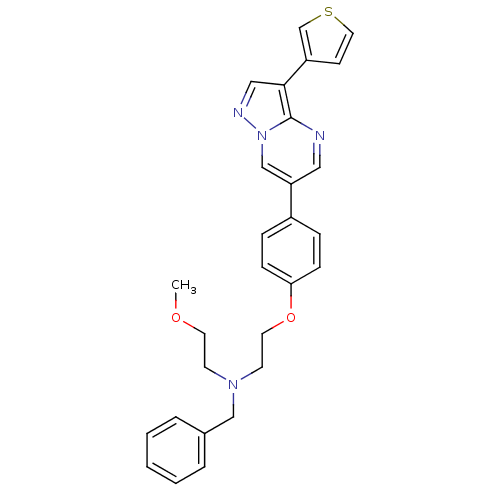 (benzyl(2-methoxyethyl)(2-{4-[3-(thiophen-3-yl)pyra...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Merck Research Laboratories | Assay Description Activated KDR was incubated with 25 uM/10 uCi of [gamma-33P] ATP, poly-Glu/Tyr, and inhibitors in kinase buffer for 15 min at 22 °C. The reactio... | Bioorg Med Chem Lett 12: 3537-41 (2002) Article DOI: 10.1016/s0960-894x(02)00827-2 BindingDB Entry DOI: 10.7270/Q2S75DHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||