

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: OP(O)(=O)C(Nc1nc(nc2sccc12)-c1cccc(NC(=O)Nc2ccc(F)cc2)c1)P(O)(O)=O
InChI Key:
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM543975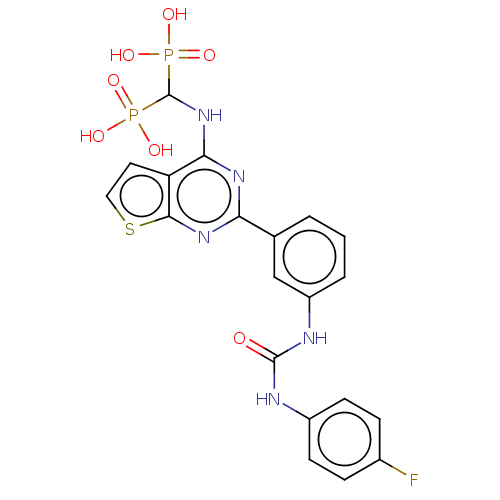 (US11279719, Example I-7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N- terminal His-tagged /TEV cleavage site fused GGPPS (1 to 300 residues) expressed in Escherichia coli BL21 (DE3) us... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01913 BindingDB Entry DOI: 10.7270/Q28K7F00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM543975 (US11279719, Example I-7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assay was based on a literature procedure (Kavanagh, et al. J. Biol. Chem., 2006, 281, 22004-22012) with minor modifications. All assays were run... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ1470 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||