

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM60438 US9067937, 28
SMILES: CN(C)C[C@H]1CC[C@@H](CC1)Nc1c(cnc2ccc(nc12)-c1cc(Cl)c(O)c(Cl)c1)C(C)=O
InChI Key: InChIKey=DKZYXHCYPUVGAF-JCNLHEQBSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maternal embryonic leucine zipper kinase (Homo sapiens (Human)) | BDBM60438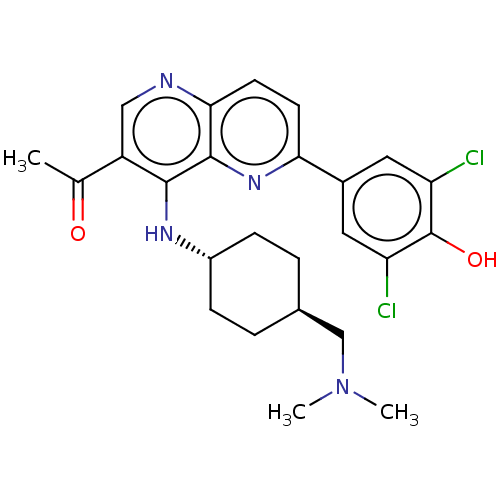 (US9067937, 28) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.4 | 25 |
OncoTherapy Science, Inc. US Patent | Assay Description MELK activity was determined in the presence or absence of compounds using fluorescein isothiocyanate-labeled (FITC-labeled) histone H3 peptide as a ... | US Patent US9067937 (2015) BindingDB Entry DOI: 10.7270/Q2BR8QX4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1A (Homo sapiens (Human)) | BDBM60438 (US9067937, 28) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
Stanford University Curated by ChEMBL | Assay Description Inhibition of human DYRK1A transfected in HEK293T cells co-transfected with Renilla plasmid, pGL3-NFAT and NFATc1 assessed as derepression of NFAT-de... | Bioorg Med Chem 28: (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maternal embryonic leucine zipper kinase (Homo sapiens (Human)) | BDBM60438 (US9067937, 28) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB PubMed | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a |
Stanford University Curated by ChEMBL | Assay Description Binding affinity to wild-type human full length MELK (M1 to V651 residues) expressed in bacterial expression system by Kinomescan method | Bioorg Med Chem 28: (2020) | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1A (Homo sapiens (Human)) | BDBM60438 (US9067937, 28) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a |
Stanford University Curated by ChEMBL | Assay Description Binding affinity to wild-type human partial length DYRK1A (H129 to S509 residues) expressed in mammalian expression system by Kinomescan method | Bioorg Med Chem 28: (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||