

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CN1CCN(CCCNc2cc3nc(NCCCN4CCN(C)CC4)c(cc3cn2)-c2ccccc2)CC1
InChI Key: InChIKey=FYWYYGLUFUROPO-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Platelet-derived growth factor receptor beta (Mus musculus (mouse)) | BDBM6160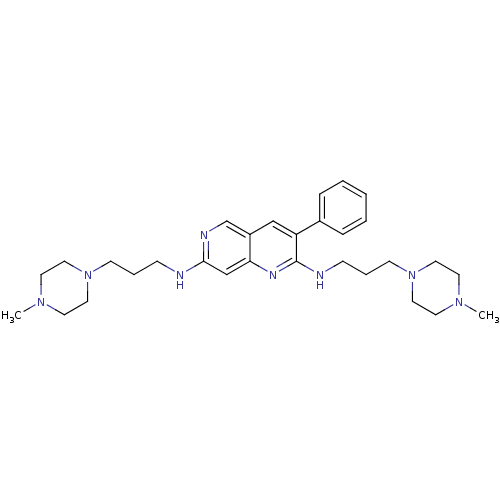 (1,6-naphthyridine 29 | 2-N,7-N-bis[3-(4-methylpipe...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description Compounds were evaluated for their ability to prevent phosphorylation of a model glutamate-tyrosine copolymer substrate by isolated human FGFR-1, mou... | J Med Chem 48: 4628-53 (2005) Article DOI: 10.1021/jm0500931 BindingDB Entry DOI: 10.7270/Q2DV1H3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM6160 (1,6-naphthyridine 29 | 2-N,7-N-bis[3-(4-methylpipe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description Tha assay was using human VEGFR-2 in DELFIA (dissociation-enhanced lanthanide fluoroimmunoassay) format. IC50 is the concentration of inhibitor that ... | J Med Chem 48: 4628-53 (2005) Article DOI: 10.1021/jm0500931 BindingDB Entry DOI: 10.7270/Q2DV1H3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM6160 (1,6-naphthyridine 29 | 2-N,7-N-bis[3-(4-methylpipe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description Compounds were evaluated for their ability to prevent phosphorylation of a model glutamate-tyrosine copolymer substrate by isolated human FGFR-1, mou... | J Med Chem 48: 4628-53 (2005) Article DOI: 10.1021/jm0500931 BindingDB Entry DOI: 10.7270/Q2DV1H3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM6160 (1,6-naphthyridine 29 | 2-N,7-N-bis[3-(4-methylpipe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description Compounds were evaluated for their ability to prevent phosphorylation of a model glutamate-tyrosine copolymer substrate by isolated human FGFR-1, mou... | J Med Chem 48: 4628-53 (2005) Article DOI: 10.1021/jm0500931 BindingDB Entry DOI: 10.7270/Q2DV1H3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||