

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CC1Cc2cc(ccc2N1C(C)=O)S(=O)(=O)N1CCN(CC1)c1ccc(cc1)[N+]([O-])=O
InChI Key: InChIKey=YPFZPYANOJYRJV-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nucleotide-binding oligomerization domain-containing protein 1 (Homo sapiens (Human)) | BDBM62245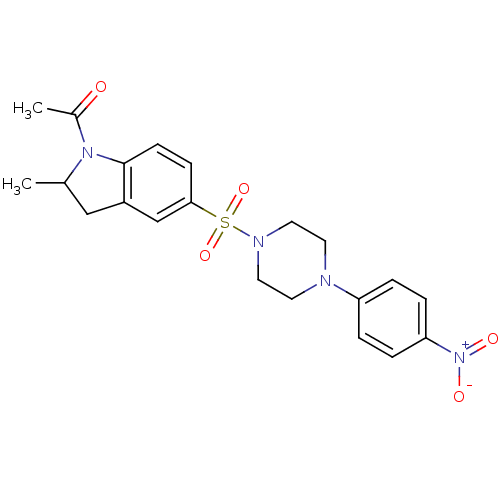 (1-[2-methyl-5-[4-(4-nitrophenyl)piperazin-1-yl]sul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2Z899VD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nucleotide-binding oligomerization domain-containing protein 1 (Homo sapiens (Human)) | BDBM62245 (1-[2-methyl-5-[4-(4-nitrophenyl)piperazin-1-yl]sul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of NOD-1 mediated NFkappaB activation in HEK293T cells assessed as inhibition of gamma-tri-DAP-induced luciferase activity after 14 hrs by... | ACS Med Chem Lett 2: 780-785 (2011) Article DOI: 10.1021/ml200158b BindingDB Entry DOI: 10.7270/Q2H9966Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nucleotide-binding oligomerization domain-containing protein 2 (Homo sapiens (Human)) | BDBM62245 (1-[2-methyl-5-[4-(4-nitrophenyl)piperazin-1-yl]sul...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of NOD-2 mediated NFkappaB activation in HEK293T cells assessed as inhibition of MDP-induced luciferase activity after 14 hrs by reporter ... | ACS Med Chem Lett 2: 780-785 (2011) Article DOI: 10.1021/ml200158b BindingDB Entry DOI: 10.7270/Q2H9966Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tumor necrosis factor (Homo sapiens (Human)) | BDBM62245 (1-[2-methyl-5-[4-(4-nitrophenyl)piperazin-1-yl]sul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 3.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2PR7TDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||