

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: Oc1ccc2cccc(NC(=O)Nc3ccccc3)c2c1
InChI Key: InChIKey=UUAPFBCLZLDKHG-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 [L188C] (Homo sapiens (Human)) | BDBM6646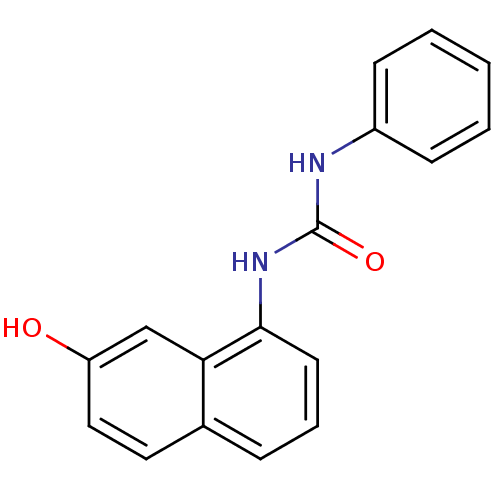 (3-(7-hydroxynaphthalen-1-yl)-1-phenylurea | Diaryl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | 30 |
Banyu Tsukuba Research Institute | Assay Description In vitro kinase assays using synthetic peptides and purified enzymes were incubated at 30°C for 45 min in buffer that contained 50 uM ATP, and d... | J Med Chem 44: 4615-27 (2001) Article DOI: 10.1021/jm0103256 BindingDB Entry DOI: 10.7270/Q2736P3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM6646 (3-(7-hydroxynaphthalen-1-yl)-1-phenylurea | Diaryl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Yakuhin, Ltd Curated by ChEMBL | Assay Description Antagonist activity at rat TRPV1 expressed in CHO cells co-expressing aequorin and CRE-luciferase reporter gene assessed as inhibition of capsaicin-i... | Bioorg Med Chem Lett 21: 3354-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.013 BindingDB Entry DOI: 10.7270/Q2NG4QXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM6646 (3-(7-hydroxynaphthalen-1-yl)-1-phenylurea | Diaryl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Yakuhin, Ltd Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells co-expressing aequorin and CRE-luciferase reporter gene assessed as inhibition of capsaicin... | Bioorg Med Chem Lett 21: 3354-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.013 BindingDB Entry DOI: 10.7270/Q2NG4QXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||