

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM7089 N-(1H-1,3-benzodiazol-2-yl)-3-{[(4R,5S,6S,7R)-3-{[3-(1H-1,3-benzodiazol-2-ylcarbamoyl)phenyl]methyl}-7-benzyl-4-ethyl-5,6-dihydroxy-2-oxo-1,3-diazepan-1-yl]methyl}benzamide::SD146 Analog 10
SMILES: CC[C@@H]1[C@H](O)[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2cccc(c2)C(=O)Nc2nc3ccccc3[nH]2)C(=O)N1Cc1cccc(c1)C(=O)Nc1nc2ccccc2[nH]1
InChI Key: InChIKey=WWYHZIBXQFYYRM-AJWAGCPHSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIV-1 Protease (Human immunodeficiency virus type 1) | BDBM7089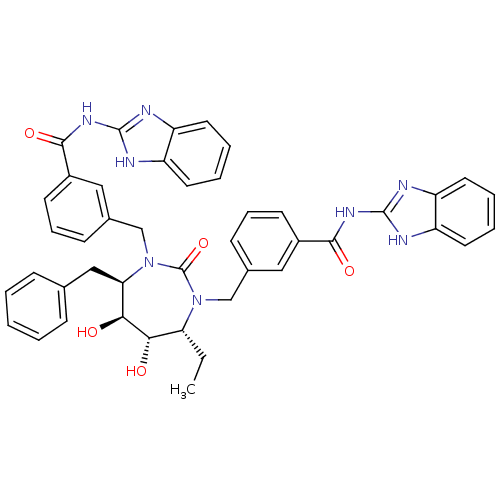 (N-(1H-1,3-benzodiazol-2-yl)-3-{[(4R,5S,6S,7R)-3-{[...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0690 | -14.4 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | Bioorg Med Chem Lett 8: 1077-82 (1998) Article DOI: 10.1016/s0960-894x(98)00175-9 BindingDB Entry DOI: 10.7270/Q21G0JGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||