

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM768 4-hydroxy-3-(1-phenylpropyl)-2H-chromen-2-one::CHEMBL16694::phenprocoumon
SMILES: CCC(c1ccccc1)c1c(O)c2ccccc2oc1=O
InChI Key: InChIKey=DQDAYGNAKTZFIW-UHFFFAOYSA-N
PDB links: 7 PDB IDs contain this monomer as substructures. 7 PDB IDs contain inhibitors having a similarity of 90% to this monomer.
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vitamin K epoxide reductase complex subunit 1 (rVKORC1) (Rattus norvegicus (Rat)) | BDBM768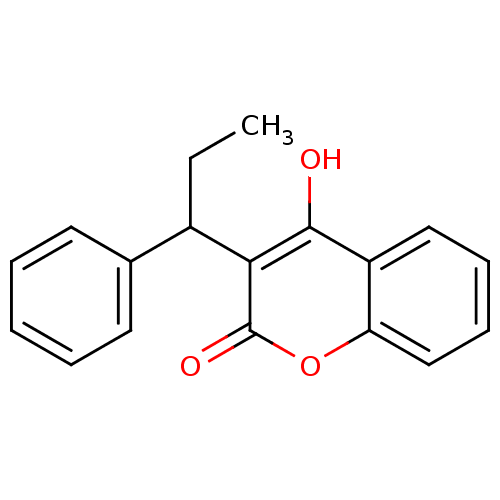 (4-hydroxy-3-(1-phenylpropyl)-2H-chromen-2-one | CH...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Claude Bernard Lyon 1 Curated by ChEMBL | Assay Description In vitro displacement of [3H]-LY 278584 from rat cerebral cortex 5-hydroxytryptamine 3 receptor | Bioorg Med Chem Lett 27: 1598-1601 (2017) Article DOI: 10.1016/j.bmcl.2017.02.017 BindingDB Entry DOI: 10.7270/Q2XP7764 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 protease (Human immunodeficiency virus type 1) | BDBM768 (4-hydroxy-3-(1-phenylpropyl)-2H-chromen-2-one | CH...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Similars | Article PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia and Upjohn Curated by ChEMBL | Assay Description Binding affinity of the compound towards HIV protease was determined | J Med Chem 39: 4125-30 (1996) Article DOI: 10.1021/jm960296c BindingDB Entry DOI: 10.7270/Q2KH0MFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 Protease (Human immunodeficiency virus type 1) | BDBM768 (4-hydroxy-3-(1-phenylpropyl)-2H-chromen-2-one | CH...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 37: 3200-4 (1994) Article DOI: 10.1021/jm00046a002 BindingDB Entry DOI: 10.7270/Q2KK98XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM768 (4-hydroxy-3-(1-phenylpropyl)-2H-chromen-2-one | CH...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.28E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 expressed in Escherichia coli JM109 | J Med Chem 49: 2417-30 (2006) Article DOI: 10.1021/jm0508538 BindingDB Entry DOI: 10.7270/Q2930TZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 protease (Human immunodeficiency virus type 1) | BDBM768 (4-hydroxy-3-(1-phenylpropyl)-2H-chromen-2-one | CH...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus 1 protease in MT-4 cells | Bioorg Med Chem Lett 15: 3257-62 (2005) Article DOI: 10.1016/j.bmcl.2005.04.057 BindingDB Entry DOI: 10.7270/Q20V8C9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||