

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM8005 (2R,3R,4R,5R)-2,5-bis({[(2E)-3-(4-acetylphenyl)prop-2-en-1-yl]oxy})-3,4-dihydroxy-N,N'-bis[(1S,2R)-2-hydroxy-2,3-dihydro-1H-inden-1-yl]hexanediamide::C2-symmetric compound 13
SMILES: CC(=O)c1ccc(\C=C\CO[C@H]([C@H](O)[C@@H](O)[C@@H](OC\C=C\c2ccc(cc2)C(C)=O)C(=O)N[C@@H]2[C@H](O)Cc3ccccc23)C(=O)N[C@@H]2[C@H](O)Cc3ccccc23)cc1
InChI Key: InChIKey=WZXYHEYYJRGWNR-VVKCFMERSA-N
Data: 6 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plasmepsin I (Plasmodium falciparum) | BDBM8005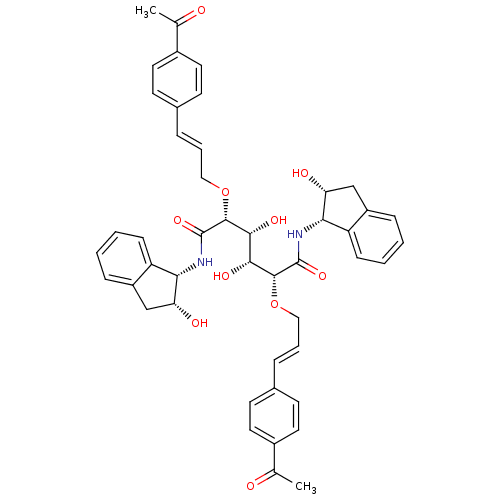 ((2R,3R,4R,5R)-2,5-bis({[(2E)-3-(4-acetylphenyl)pro...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 110-22 (2004) Article DOI: 10.1021/jm030933g BindingDB Entry DOI: 10.7270/Q2RV0KX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM8005 ((2R,3R,4R,5R)-2,5-bis({[(2E)-3-(4-acetylphenyl)pro...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zambia Curated by ChEMBL | Assay Description Inhibition of recombinant Plasmodium falciparum Plasmepsin 1 | J Med Chem 63: 4445-4467 (2020) Article DOI: 10.1021/acs.jmedchem.9b01622 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin 2 (Plasmodium falciparum) | BDBM8005 ((2R,3R,4R,5R)-2,5-bis({[(2E)-3-(4-acetylphenyl)pro...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6 | -11.1 | n/a | n/a | n/a | n/a | n/a | 4.5 | 22 |
Uppsala University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 110-22 (2004) Article DOI: 10.1021/jm030933g BindingDB Entry DOI: 10.7270/Q2RV0KX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin 2 (Plasmodium falciparum) | BDBM8005 ((2R,3R,4R,5R)-2,5-bis({[(2E)-3-(4-acetylphenyl)pro...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zambia Curated by ChEMBL | Assay Description Inhibition of recombinant Plasmodium falciparum Plasmepsin 2 | J Med Chem 63: 4445-4467 (2020) Article DOI: 10.1021/acs.jmedchem.9b01622 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM8005 ((2R,3R,4R,5R)-2,5-bis({[(2E)-3-(4-acetylphenyl)pro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zambia Curated by ChEMBL | Assay Description Inhibition of human cathepsin D | J Med Chem 63: 4445-4467 (2020) Article DOI: 10.1021/acs.jmedchem.9b01622 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM8005 ((2R,3R,4R,5R)-2,5-bis({[(2E)-3-(4-acetylphenyl)pro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 110-22 (2004) Article DOI: 10.1021/jm030933g BindingDB Entry DOI: 10.7270/Q2RV0KX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||