

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM8181 N-(2,3-dihydro-1,4-benzodioxin-6-yl)-4-[2-(4-methoxyphenyl)pyrazolo[1,5-a]pyridazin-3-yl]pyrimidin-2-amine::pyrazolo[1,5-b]pyridazine deriv. 72
SMILES: COc1ccc(cc1)-c1nn2ncccc2c1-c1ccnc(Nc2ccc3OCCOc3c2)n1
InChI Key: InChIKey=PHMQSKGFSDGFMK-UHFFFAOYSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cyclin-Dependent Kinase 2 (CDK2) (Homo sapiens (Human)) | BDBM8181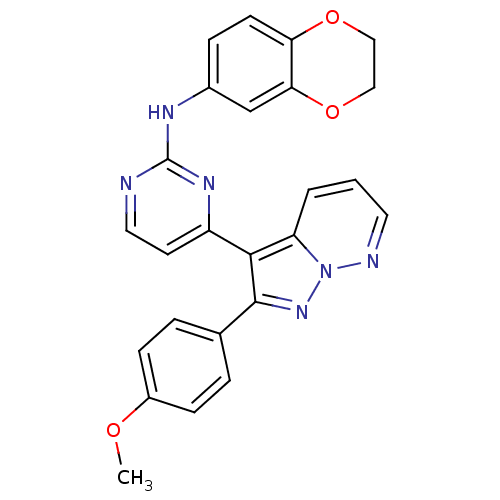 (N-(2,3-dihydro-1,4-benzodioxin-6-yl)-4-[2-(4-metho...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8181 (N-(2,3-dihydro-1,4-benzodioxin-6-yl)-4-[2-(4-metho...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human GSK3beta using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate af... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM8181 (N-(2,3-dihydro-1,4-benzodioxin-6-yl)-4-[2-(4-metho...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | <2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human CDK2 using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate after ... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8181 (N-(2,3-dihydro-1,4-benzodioxin-6-yl)-4-[2-(4-metho...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||