 Found 7 hits for monomerid = 8466
Found 7 hits for monomerid = 8466 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM8466
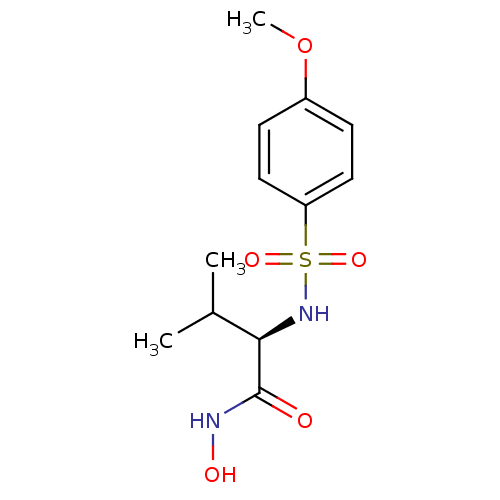
((2R)-N-hydroxy-2-[(4-methoxybenzene)sulfonamido]-3...)Show SMILES COc1ccc(cc1)S(=O)(=O)N[C@H](C(C)C)C(=O)NO |r| Show InChI InChI=1S/C12H18N2O5S/c1-8(2)11(12(15)13-16)14-20(17,18)10-6-4-9(19-3)5-7-10/h4-8,11,14,16H,1-3H3,(H,13,15)/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pomona College
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem 15: 2223-68 (2007)
Article DOI: 10.1016/j.bmc.2007.01.011
BindingDB Entry DOI: 10.7270/Q2571DBD |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM8466

((2R)-N-hydroxy-2-[(4-methoxybenzene)sulfonamido]-3...)Show SMILES COc1ccc(cc1)S(=O)(=O)N[C@H](C(C)C)C(=O)NO |r| Show InChI InChI=1S/C12H18N2O5S/c1-8(2)11(12(15)13-16)14-20(17,18)10-6-4-9(19-3)5-7-10/h4-8,11,14,16H,1-3H3,(H,13,15)/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pomona College
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem 15: 2223-68 (2007)
Article DOI: 10.1016/j.bmc.2007.01.011
BindingDB Entry DOI: 10.7270/Q2571DBD |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM8466

((2R)-N-hydroxy-2-[(4-methoxybenzene)sulfonamido]-3...)Show SMILES COc1ccc(cc1)S(=O)(=O)N[C@H](C(C)C)C(=O)NO |r| Show InChI InChI=1S/C12H18N2O5S/c1-8(2)11(12(15)13-16)14-20(17,18)10-6-4-9(19-3)5-7-10/h4-8,11,14,16H,1-3H3,(H,13,15)/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human matrix metalloprotease-1 (MMP-1) |
Bioorg Med Chem Lett 14: 3389-95 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.083
BindingDB Entry DOI: 10.7270/Q25Q4WPN |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM8466

((2R)-N-hydroxy-2-[(4-methoxybenzene)sulfonamido]-3...)Show SMILES COc1ccc(cc1)S(=O)(=O)N[C@H](C(C)C)C(=O)NO |r| Show InChI InChI=1S/C12H18N2O5S/c1-8(2)11(12(15)13-16)14-20(17,18)10-6-4-9(19-3)5-7-10/h4-8,11,14,16H,1-3H3,(H,13,15)/t11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-13 (MMP-13) |
Bioorg Med Chem Lett 14: 3389-95 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.083
BindingDB Entry DOI: 10.7270/Q25Q4WPN |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM8466

((2R)-N-hydroxy-2-[(4-methoxybenzene)sulfonamido]-3...)Show SMILES COc1ccc(cc1)S(=O)(=O)N[C@H](C(C)C)C(=O)NO |r| Show InChI InChI=1S/C12H18N2O5S/c1-8(2)11(12(15)13-16)14-20(17,18)10-6-4-9(19-3)5-7-10/h4-8,11,14,16H,1-3H3,(H,13,15)/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Binding affinity to stromelysin-1 catalytic domain expressed in Escherichia coli BL21 (DE3) by isothermal titration colorimetry |
Bioorg Med Chem Lett 20: 280-2 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.114
BindingDB Entry DOI: 10.7270/Q20V8DQN |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM8466

((2R)-N-hydroxy-2-[(4-methoxybenzene)sulfonamido]-3...)Show SMILES COc1ccc(cc1)S(=O)(=O)N[C@H](C(C)C)C(=O)NO |r| Show InChI InChI=1S/C12H18N2O5S/c1-8(2)11(12(15)13-16)14-20(17,18)10-6-4-9(19-3)5-7-10/h4-8,11,14,16H,1-3H3,(H,13,15)/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 113 | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Binding affinity to stromelysin-1 catalytic domain |
Bioorg Med Chem Lett 20: 280-2 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.114
BindingDB Entry DOI: 10.7270/Q20V8DQN |
More data for this
Ligand-Target Pair | |
Anthrax Lethal Factor (LF)
(Bacillus anthracis) | BDBM8466

((2R)-N-hydroxy-2-[(4-methoxybenzene)sulfonamido]-3...)Show SMILES COc1ccc(cc1)S(=O)(=O)N[C@H](C(C)C)C(=O)NO |r| Show InChI InChI=1S/C12H18N2O5S/c1-8(2)11(12(15)13-16)14-20(17,18)10-6-4-9(19-3)5-7-10/h4-8,11,14,16H,1-3H3,(H,13,15)/t11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Merck Research Laboratories
| Assay Description
The assay was performed in a 96-well plate, each well contained substrate peptide, LF, and the test compound. The C-terminally fluorophore of substra... |
Bioorg Med Chem Lett 16: 964-8 (2006)
Article DOI: 10.1016/j.bmcl.2005.10.088
BindingDB Entry DOI: 10.7270/Q2K35RV4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data