

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM85154 2,3,4,5-Tetrahydro-3-(1H-indazole-3-ylmethyl)-N-(4-methoxyphenyl)-N-(1-methylethyl)-2,4-dioxo-5-phenyl-1H-1,5-benzodiazepine-1-acetamide::CCK-A Agonist 1::GW 5823::GW-5823::N-Isopropyl-N-(4-methoxyphenyl)-3-(1H-indazol-3-ylmethyl)-5-phenyl-2,4-dioxo-2,3,4,5-tetrahydro-1H-1,5-benzodiazepine-1-acetamide
SMILES: COc1ccc(cc1)N(C(C)C)C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)C(Cc2n[nH]c3ccccc23)C1=O
InChI Key: InChIKey=LGYKPDHARJMLQN-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholecystokinin receptor (Homo sapiens (Human)) | BDBM85154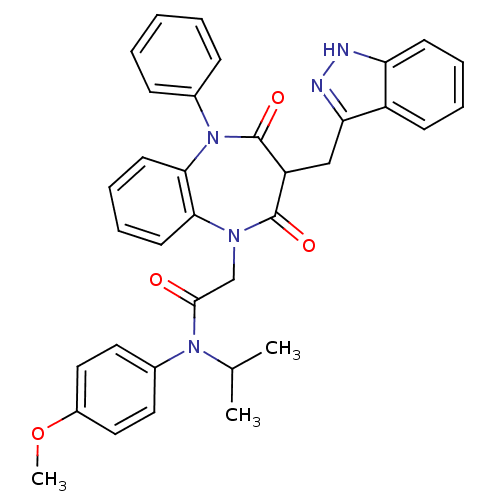 (2,3,4,5-Tetrahydro-3-(1H-indazole-3-ylmethyl)-N-(4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by PDSP Ki Database | J Med Chem 40: 2706-25 (1997) Article DOI: 10.1021/jm970265x BindingDB Entry DOI: 10.7270/Q27M06F9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor (Homo sapiens (Human)) | BDBM85154 (2,3,4,5-Tetrahydro-3-(1H-indazole-3-ylmethyl)-N-(4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Binding affinity against human Cholecystokinin type B receptor by displacement of [125I]CCK-8 | J Med Chem 39: 2655-8 (1996) Article DOI: 10.1021/jm960249k BindingDB Entry DOI: 10.7270/Q2JQ126Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor (Homo sapiens (Human)) | BDBM85154 (2,3,4,5-Tetrahydro-3-(1H-indazole-3-ylmethyl)-N-(4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Binding affinity against human Cholecystokinin type A receptor by displacement of [125I]CCK-8 | J Med Chem 39: 2655-8 (1996) Article DOI: 10.1021/jm960249k BindingDB Entry DOI: 10.7270/Q2JQ126Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||