

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM855 (2R,3R,4R,5R)-3,4-dihydroxy-2,5-bis({[4-(2-hydroxy-3-oxocyclohex-1-en-1-yl)phenyl]methoxy})-N,N'-bis[(1S)-2-methyl-1-(methylcarbamoyl)propyl]hexanediamide::C2-Symmetric inhibitor 13::CHEMBL338428::N1,N6-Bis[(1S)-2-methyl-1-(methylcarbamoyl)pro-pyl](2R,3R,4R,5R)-2,5-bis[4-(3-cyclohexane-1,2-dionyl)benzyloxy]-3,4-dihydroxyhexanediamide
SMILES: CNC(=O)[C@@H](NC(=O)[C@H](OCc1ccc(cc1)C1CCCC(=O)C1=O)[C@H](O)[C@@H](O)[C@@H](OCc1ccc(cc1)C1CCCC(=O)C1=O)C(=O)N[C@@H](C(C)C)C(=O)NC)C(C)C
InChI Key: InChIKey=FNBWPIBLHMWNLO-SSZBCMKESA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIV-1 Protease (Human immunodeficiency virus type 1) | BDBM855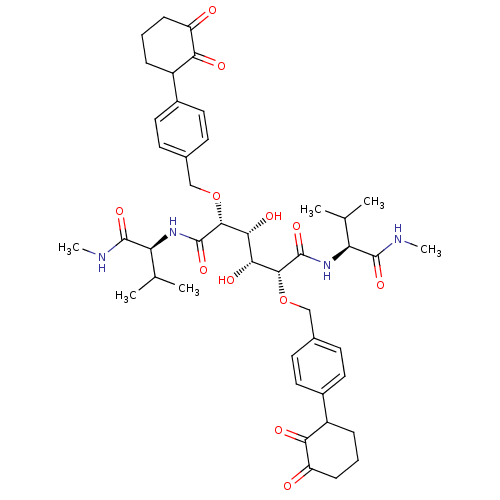 ((2R,3R,4R,5R)-3,4-dihydroxy-2,5-bis({[4-(2-hydroxy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | -13.2 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Uppsala University | Assay Description Ki values were determined by using a fluorescent substrate (DABCYL-gamma-Abu-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-EDANS). All incubations were performed a... | J Med Chem 42: 3835-44 (1999) Article DOI: 10.1021/jm9910371 BindingDB Entry DOI: 10.7270/Q2T151VX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 protease (Human immunodeficiency virus type 1) | BDBM855 ((2R,3R,4R,5R)-3,4-dihydroxy-2,5-bis({[4-(2-hydroxy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.300 | n/a | n/a | 18 | n/a | n/a | 2.93E+4 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Association rate constant for the interaction between inhibitor and HIV-1 protease | J Med Chem 45: 5430-9 (2002) BindingDB Entry DOI: 10.7270/Q2GH9JP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 protease (Human immunodeficiency virus type 1) | BDBM855 ((2R,3R,4R,5R)-3,4-dihydroxy-2,5-bis({[4-(2-hydroxy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | n/a | 0.000487 | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Association rate constant for the interaction between inhibitor and HIV-1 protease | J Med Chem 45: 5430-9 (2002) BindingDB Entry DOI: 10.7270/Q2GH9JP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||