 Found 6 hits for monomerid = 8579
Found 6 hits for monomerid = 8579 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM8579
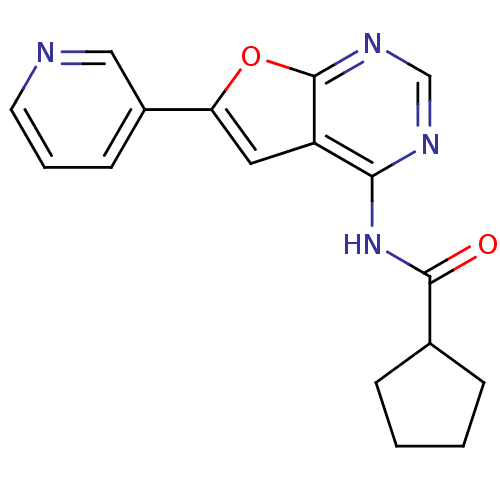
(4-Acylamino-6-arylfuro[2,3-d]pyrimidine 24 | N-[6-...)Show InChI InChI=1S/C17H16N4O2/c22-16(11-4-1-2-5-11)21-15-13-8-14(12-6-3-7-18-9-12)23-17(13)20-10-19-15/h3,6-11H,1-2,4-5H2,(H,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Tsukuba Research Laboratories
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... |
Bioorg Med Chem Lett 14: 3907-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.064
BindingDB Entry DOI: 10.7270/Q2XD0ZWC |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM8579

(4-Acylamino-6-arylfuro[2,3-d]pyrimidine 24 | N-[6-...)Show InChI InChI=1S/C17H16N4O2/c22-16(11-4-1-2-5-11)21-15-13-8-14(12-6-3-7-18-9-12)23-17(13)20-10-19-15/h3,6-11H,1-2,4-5H2,(H,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Laboratories
| Assay Description
The assay was using baculovirus-expressed recombinant protein kinase purified as the intracellular domain fused by GST tag, interacting with biotinyl... |
Bioorg Med Chem Lett 14: 3907-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.064
BindingDB Entry DOI: 10.7270/Q2XD0ZWC |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM8579

(4-Acylamino-6-arylfuro[2,3-d]pyrimidine 24 | N-[6-...)Show InChI InChI=1S/C17H16N4O2/c22-16(11-4-1-2-5-11)21-15-13-8-14(12-6-3-7-18-9-12)23-17(13)20-10-19-15/h3,6-11H,1-2,4-5H2,(H,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of human Protein kinase C alpha |
Eur J Med Chem 164: 448-470 (2019)
Article DOI: 10.1007/s00044-005-0126-y
BindingDB Entry DOI: 10.7270/Q2WH2SVB |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM8579

(4-Acylamino-6-arylfuro[2,3-d]pyrimidine 24 | N-[6-...)Show InChI InChI=1S/C17H16N4O2/c22-16(11-4-1-2-5-11)21-15-13-8-14(12-6-3-7-18-9-12)23-17(13)20-10-19-15/h3,6-11H,1-2,4-5H2,(H,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Road
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta |
Eur J Med Chem 44: 2361-71 (2009)
Article DOI: 10.1016/j.ejmech.2008.08.012
BindingDB Entry DOI: 10.7270/Q2057FZX |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM8579

(4-Acylamino-6-arylfuro[2,3-d]pyrimidine 24 | N-[6-...)Show InChI InChI=1S/C17H16N4O2/c22-16(11-4-1-2-5-11)21-15-13-8-14(12-6-3-7-18-9-12)23-17(13)20-10-19-15/h3,6-11H,1-2,4-5H2,(H,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta by scintillation proximity assay |
Eur J Med Chem 144: 843-858 (2018)
Article DOI: 10.1016/j.ejmech.2017.11.103
BindingDB Entry DOI: 10.7270/Q2Q242SG |
More data for this
Ligand-Target Pair | |
Cyclin-Dependent Kinase 2 (CDK2)
(Homo sapiens (Human)) | BDBM8579

(4-Acylamino-6-arylfuro[2,3-d]pyrimidine 24 | N-[6-...)Show InChI InChI=1S/C17H16N4O2/c22-16(11-4-1-2-5-11)21-15-13-8-14(12-6-3-7-18-9-12)23-17(13)20-10-19-15/h3,6-11H,1-2,4-5H2,(H,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 457 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Laboratories
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A... |
Bioorg Med Chem Lett 14: 3907-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.064
BindingDB Entry DOI: 10.7270/Q2XD0ZWC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data