

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM860 1OH-2(Cbz-Tle)3PhPr [14]paracyclophane deriv. 25::tert-butyl N-[(1S,2S)-1-[(9R,12R)-8,11-dioxo-9-(propan-2-yl)-2-oxa-7,10,13-triazabicyclo[13.2.2]nonadeca-1(17),15,18-trien-12-yl]-1-hydroxy-3-phenylpropan-2-yl]carbamate
SMILES: CC(C)C1NC(=O)[C@H](NCc2ccc(OCCCCNC1=O)cc2)C(O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C
InChI Key: InChIKey=AOOUKNIZXIPRPV-JCYSWHRNSA-N
Data: 1 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIV-1 Protease (Human immunodeficiency virus type 1) | BDBM860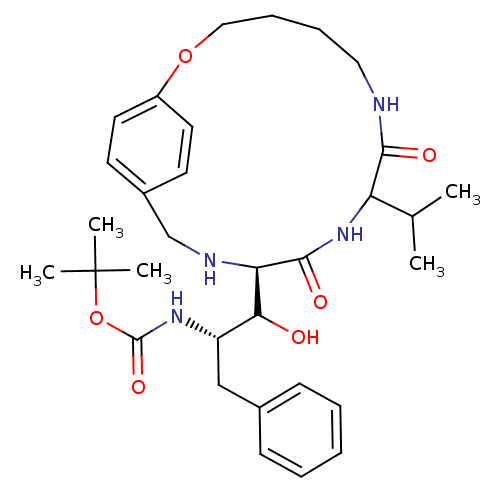 (1OH-2(Cbz-Tle)3PhPr [14]paracyclophane deriv. 25 |...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 17 | -11.0 | n/a | n/a | n/a | n/a | n/a | 6.25 | 37 |
Sandoz Research Institute | Assay Description Enzymatic activity was measured by following cleavage of the substrate H-Lys-Ala-Arg-Val-Leu-pNph-Glu-Ala-Nle-NH2. Products of the cleavage reaction ... | J Med Chem 39: 3291-9 (1996) Article DOI: 10.1021/jm950641i BindingDB Entry DOI: 10.7270/Q2P84935 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||