

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM86010 Chalcone, 10
SMILES: Oc1ccc(Cl)cc1C(=O)\C=C\c1ccccc1
InChI Key: InChIKey=GVKYSLWFMZCXBQ-SOFGYWHQSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lipoxygenase (Homo sapiens (Human)) | BDBM86010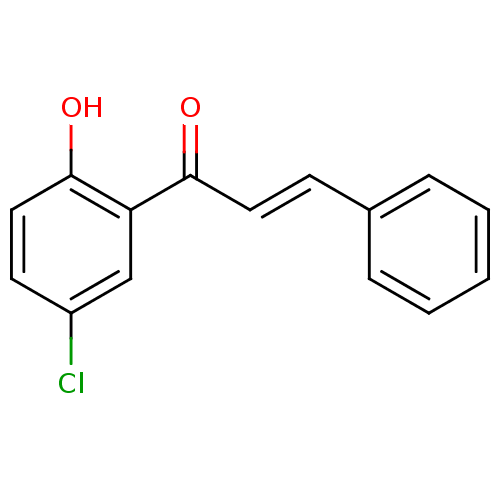 (Chalcone, 10) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 5.76E+4 | -5.78 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Quaid-i-Azam University | Assay Description In vitro lipoxygenase inhibition assay, lipoxygenase inhibiting activity was convenintly measured by slightly modifying the spectrometic method devel... | J Enzyme Inhib Med Chem 20: 41-7 (2005) Article DOI: 10.1080/14756360400015231 BindingDB Entry DOI: 10.7270/Q2FX781C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldose reductase (Rattus norvegicus) | BDBM86010 (Chalcone, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Rattus norvegicus (rat) lens aldose reductase | Citation and Details Article DOI: 10.1007/s00044-012-0367-5 BindingDB Entry DOI: 10.7270/Q2XP77VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen Receptor (Homo sapiens (Human)) | BDBM86010 (Chalcone, 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kanazawa University Curated by ChEMBL | Assay Description Antagonist activity at AR in human LNCAP cells assessed as reduction in DHT-induced transcriptional activation after 24 hrs by luciferase reporter ge... | Eur J Med Chem 157: 1143-1152 (2018) Article DOI: 10.1016/j.ejmech.2018.08.069 BindingDB Entry DOI: 10.7270/Q24X5BHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM86010 (Chalcone, 10) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.92E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Quaid-i-Azam University | Assay Description In vitro cholinesterase inhibition assay using electric-eel acetylcholinesterase, horse-serum butyrylcholinesterse. The IC50 values were calculated ... | J Enzyme Inhib Med Chem 20: 41-7 (2005) Article DOI: 10.1080/14756360400015231 BindingDB Entry DOI: 10.7270/Q2FX781C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||