

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2=CC(=O)CC[C@]12C
InChI Key: InChIKey=MUMGGOZAMZWBJJ-DYKIIFRCSA-N
PDB links: 32 PDB IDs match this monomer. 4 PDB IDs contain this monomer as substructures. 4 PDB IDs contain inhibitors having a similarity of 90% to this monomer.
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Androgen receptor (Homo sapiens (Human)) | BDBM8885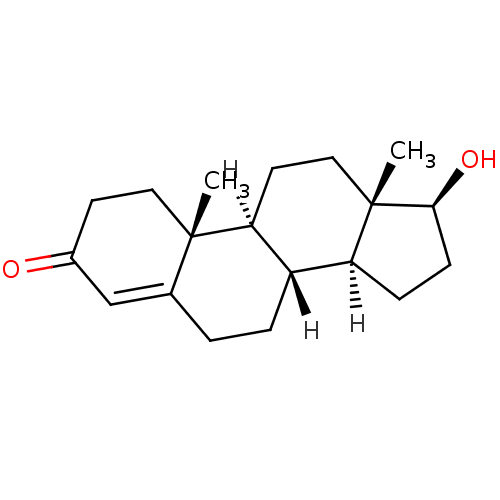 ((1S,2R,10R,11S,14S,15S)-14-hydroxy-2,15-dimethylte...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Gothenburg Curated by ChEMBL | Assay Description Antagonist activity against pSG5-tagged human androgen receptor expressed in COS1 cells assessed as reduction in receptor-mediated transcriptional ac... | J Med Chem 58: 1569-74 (2015) Article DOI: 10.1021/jm501995n BindingDB Entry DOI: 10.7270/Q24X59G2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Mus musculus) | BDBM8885 ((1S,2R,10R,11S,14S,15S)-14-hydroxy-2,15-dimethylte...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.4 | 25 |
ENDORECHERCHE, INC. US Patent | Assay Description Androgen binding is measured using the hydroxylapatite (HAP) assay. In brief, the radioactive steroid [3H]R1881 solubilized in ethanol is diluted wit... | US Patent US9682960 (2017) BindingDB Entry DOI: 10.7270/Q2PZ5708 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| UDP-glucuronosyltransferase 2B10 (Homo sapiens (Human)) | BDBM8885 ((1S,2R,10R,11S,14S,15S)-14-hydroxy-2,15-dimethylte...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences | Assay Description Refer to Fisher et al., Drug Metab. Dispos., 28:560-566. | J Enzyme Inhib Med Chem 26: 386-93 (2011) Article DOI: 10.3109/14756366.2010.518965 BindingDB Entry DOI: 10.7270/Q2HQ3XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 2B7 (Homo sapiens (Human)) | BDBM8885 ((1S,2R,10R,11S,14S,15S)-14-hydroxy-2,15-dimethylte...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.65E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences | Assay Description Refer to Fisher et al., Drug Metab. Dispos., 28:560-566. | J Enzyme Inhib Med Chem 26: 386-93 (2011) Article DOI: 10.3109/14756366.2010.518965 BindingDB Entry DOI: 10.7270/Q2HQ3XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1-6 (Homo sapiens (Human)) | BDBM8885 ((1S,2R,10R,11S,14S,15S)-14-hydroxy-2,15-dimethylte...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences | Assay Description Refer to Fisher et al., Drug Metab. Dispos., 28:560-566. | J Enzyme Inhib Med Chem 26: 386-93 (2011) Article DOI: 10.3109/14756366.2010.518965 BindingDB Entry DOI: 10.7270/Q2HQ3XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM8885 ((1S,2R,10R,11S,14S,15S)-14-hydroxy-2,15-dimethylte...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences | Assay Description Refer to Fisher et al., Drug Metab. Dispos., 28:560-566. | J Enzyme Inhib Med Chem 26: 386-93 (2011) Article DOI: 10.3109/14756366.2010.518965 BindingDB Entry DOI: 10.7270/Q2HQ3XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8885 ((1S,2R,10R,11S,14S,15S)-14-hydroxy-2,15-dimethylte...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 5.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jordan | Assay Description AChE and BChE inhibiting activities were measured in vitro by a modified spectrophotometric method previously developed by Ellman et. al. | J Enzyme Inhib Med Chem 24: 553-8 (2009) Article DOI: 10.1080/14756360802236393 BindingDB Entry DOI: 10.7270/Q20K274X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM8885 ((1S,2R,10R,11S,14S,15S)-14-hydroxy-2,15-dimethylte...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 4.57 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Eastern Finland Curated by ChEMBL | Assay Description Displacement of [3H]R1881 from wild type human androgen receptor expressed in COS-1 cells co-transfected with pSG5 after 15 mins by scintillation ass... | J Med Chem 55: 6316-27 (2012) Article DOI: 10.1021/jm300233k BindingDB Entry DOI: 10.7270/Q2NS0W1N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| << First | Previous | Displayed 51 to 58 (of 58 total ) |