

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM91679 Pyrazolyl benzenesulfonamide derivative, 7b
SMILES: Cc1ccc(cc1)-c1nn(cc1C(N)=O)-c1ccc(cc1)S(N)(=O)=O
InChI Key: InChIKey=KQNLJPQBNLBZFU-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase (Homo sapiens (Human)) | BDBM91679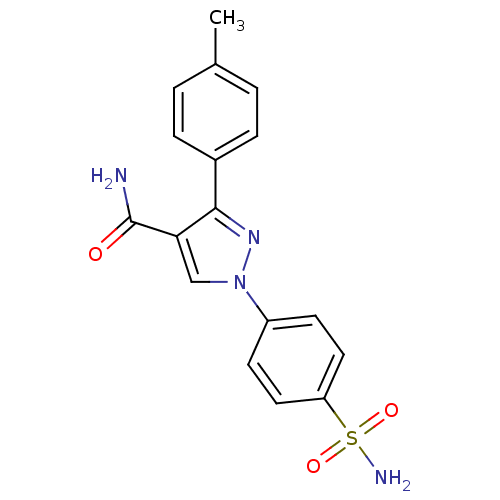 (Pyrazolyl benzenesulfonamide derivative, 7b) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kurukshetra University Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 12-mediated CO2 hydration preincubated for 10 mins by stopped-flow assay | Eur J Med Chem 76: 284-90 (2014) Article DOI: 10.1016/j.ejmech.2014.02.023 BindingDB Entry DOI: 10.7270/Q2833W01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrases; II & IX (Homo sapiens (Human)) | BDBM91679 (Pyrazolyl benzenesulfonamide derivative, 7b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kurukshetra University Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 9-mediated CO2 hydration preincubated for 10 mins by stopped-flow assay | Eur J Med Chem 76: 284-90 (2014) Article DOI: 10.1016/j.ejmech.2014.02.023 BindingDB Entry DOI: 10.7270/Q2833W01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM91679 (Pyrazolyl benzenesulfonamide derivative, 7b) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kurukshetra University Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 2-mediated CO2 hydration preincubated for 10 mins by stopped-flow assay | Eur J Med Chem 76: 284-90 (2014) Article DOI: 10.1016/j.ejmech.2014.02.023 BindingDB Entry DOI: 10.7270/Q2833W01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase (Homo sapiens (Human)) | BDBM91679 (Pyrazolyl benzenesulfonamide derivative, 7b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kurukshetra University Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 1-mediated CO2 hydration preincubated for 10 mins by stopped-flow assay | Eur J Med Chem 76: 284-90 (2014) Article DOI: 10.1016/j.ejmech.2014.02.023 BindingDB Entry DOI: 10.7270/Q2833W01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclooxygenase (Homo sapiens (Human)) | BDBM91679 (Pyrazolyl benzenesulfonamide derivative, 7b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alexandria | Assay Description The compounds that exhibited potent anti-inflammatory profiles were futher tested for their ability to inhibit human COX-1 and COX-2 enzymes in-vitro... | J Enzyme Inhib Med Chem 24: 296-309 (2009) Article DOI: 10.1080/14756360802188404 BindingDB Entry DOI: 10.7270/Q2S1813W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclooxygenase (Homo sapiens (Human)) | BDBM91679 (Pyrazolyl benzenesulfonamide derivative, 7b) | MMDB Reactome pathway B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alexandria | Assay Description The compounds that exhibited potent anti-inflammatory profiles were futher tested for their ability to inhibit human COX-1 and COX-2 enzymes in-vitro... | J Enzyme Inhib Med Chem 24: 296-309 (2009) Article DOI: 10.1080/14756360802188404 BindingDB Entry DOI: 10.7270/Q2S1813W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||