

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM91716 11alpha-Hydroxyandrost-4-en-3,17-dione, 5
SMILES: C[C@]12CC[C@@]3(O)[C@@H](CCC4=CC(=O)CC[C@]34C)[C@@H]1CCC2=O
InChI Key: InChIKey=SNMVJSSWZSJOGL-PLOWYNNNSA-N
Data: 1 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterases (Homo sapiens (Human)) | BDBM91716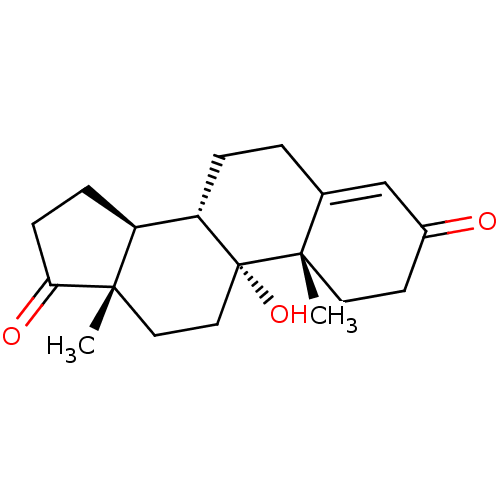 (11alpha-Hydroxyandrost-4-en-3,17-dione, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.41E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jordan | Assay Description AChE and BChE inhibiting activities were measured in vitro by a modified spectrophotometric method previously developed by Ellman et. al. | J Enzyme Inhib Med Chem 24: 553-8 (2009) Article DOI: 10.1080/14756360802236393 BindingDB Entry DOI: 10.7270/Q20K274X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||