

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM942 5PhBuCOOH deriv.::Statine deriv. 22::Tle-Val-Sta::benzyl N-[(1S)-1-{[(2S,3R,4R)-4-(benzylamino)-4-{[(1S)-1-(benzylcarbamoyl)-2-methylpropyl]carbamoyl}-3-hydroxy-1-phenylbutan-2-yl]carbamoyl}-2,2-dimethylpropyl]carbamate
SMILES: CC(C)[C@H](NC(=O)[C@H](NCc1ccccc1)[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)(C)C)C(=O)NCc1ccccc1
InChI Key: InChIKey=FLCJUYJOFGNZSU-VEJIKZNJSA-N
Data: 3 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Human immunodeficiency virus type 1 protease (Human immunodeficiency virus type 1) | BDBM942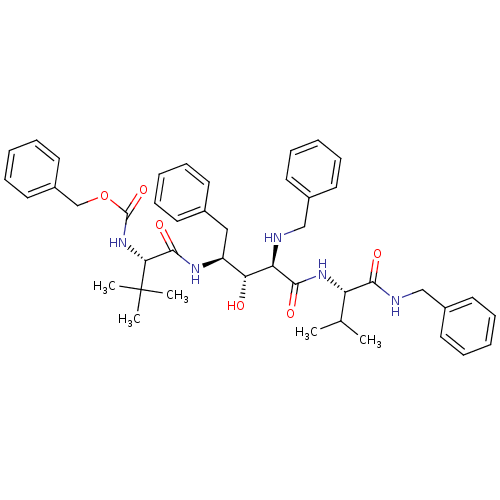 (5PhBuCOOH deriv. | Statine deriv. 22 | Tle-Val-Sta...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 9.12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SANDOZ Forschungsinstitut Ges. m.b.H Curated by ChEMBL | Assay Description Inhibitory activity was determined against HIV type 1 protease | J Med Chem 38: 4917-28 (1996) BindingDB Entry DOI: 10.7270/Q2FX7BN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 Protease (Human immunodeficiency virus type 1) | BDBM942 (5PhBuCOOH deriv. | Statine deriv. 22 | Tle-Val-Sta...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9.20 | -11.4 | n/a | n/a | n/a | n/a | n/a | 6.25 | 37 |
SANDOZ Forschungsinstitut Ges.m.b.H. | Assay Description Enzymatic activity was measured by following cleavage of the substrate H-Lys-Ala-Arg-Val-Leu-pNph-Glu-Ala-Nle-NH2. Products of the cleavage reaction ... | J Med Chem 37: 3079-89 (1994) Article DOI: 10.1021/jm00045a013 BindingDB Entry DOI: 10.7270/Q21J97XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-2 Protease (Human immunodeficiency virus type 2) | BDBM942 (5PhBuCOOH deriv. | Statine deriv. 22 | Tle-Val-Sta...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 54 | -10.3 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
SANDOZ Forschungsinstitut Ges.m.b.H. | Assay Description Enzymatic activity was measured by following cleavage of the substrate H-Lys-Ala-Arg-Val-Leu-pNph-Glu-Ala-Nle-NH2. Products of the cleavage reaction ... | J Med Chem 37: 3079-89 (1994) Article DOI: 10.1021/jm00045a013 BindingDB Entry DOI: 10.7270/Q21J97XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||