

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: c1cn(cn1)C1(c2ccccc2-c2ccccc12)c1ccccc1
InChI Key: InChIKey=DNCKVRPGVMTVNH-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aromatase (Homo sapiens (Human)) | BDBM9465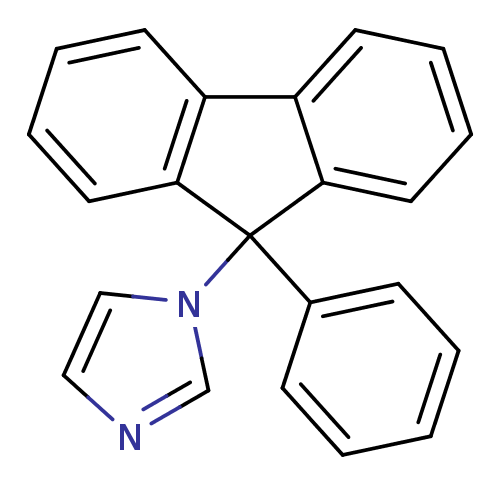 (1-(9-Phenyl-9H-fluoren-9-yl)-1H-imidazole | CHEMBL...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bari | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9465 (1-(9-Phenyl-9H-fluoren-9-yl)-1H-imidazole | CHEMBL...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University Curated by ChEMBL | Assay Description Inhibition of human placental microsome CYP19 | Bioorg Med Chem Lett 20: 3050-64 (2010) Article DOI: 10.1016/j.bmcl.2010.03.113 BindingDB Entry DOI: 10.7270/Q2CJ8FFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9465 (1-(9-Phenyl-9H-fluoren-9-yl)-1H-imidazole | CHEMBL...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Coimbra Curated by ChEMBL | Assay Description Inhibition of human aromatase-mediated conversion of [1beta3H]androstenedione to estrone by liquid scintillation counting in presence of NADPH | J Med Chem 52: 143-50 (2009) Article DOI: 10.1021/jm800945c BindingDB Entry DOI: 10.7270/Q2SQ9080 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM9465 (1-(9-Phenyl-9H-fluoren-9-yl)-1H-imidazole | CHEMBL...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||