

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Wt: 354.4 BDBM133985  | Wt: 341.4 BDBM133995  | Wt: 341.4 BDBM133996 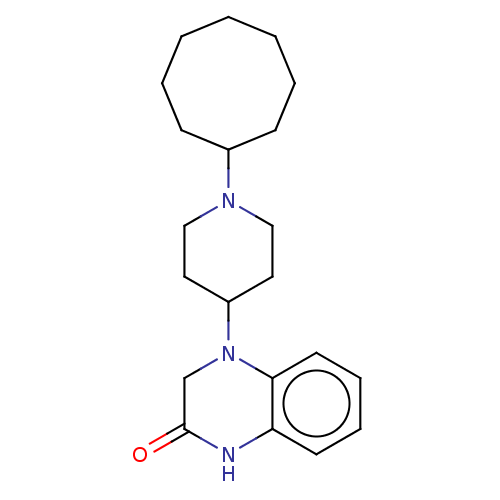 | Wt: 364.4 BDBM133912  | Wt: 369.4 BDBM18576  |
| Wt: 374.5 BDBM177916 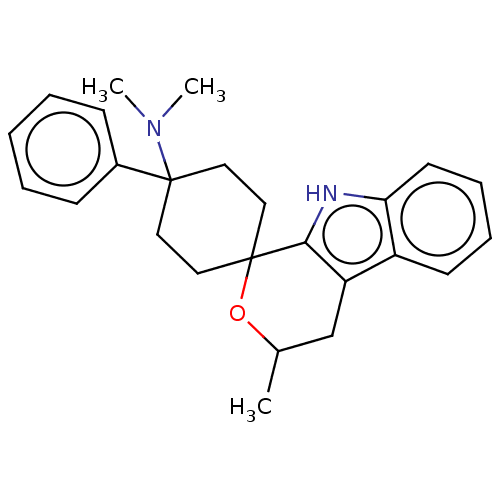 | Wt: 359.5 BDBM177911 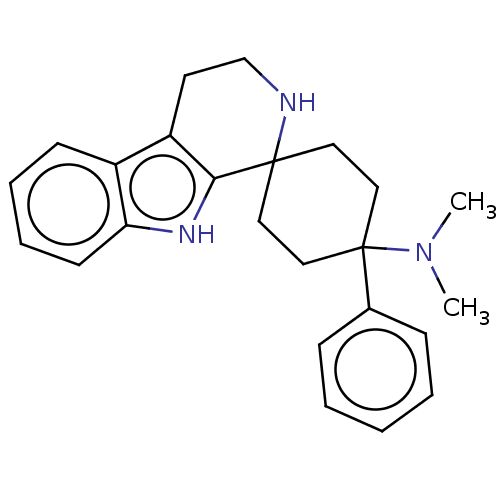 | Wt: 360.4 BDBM177903  | Wt: 374.5 BDBM177928 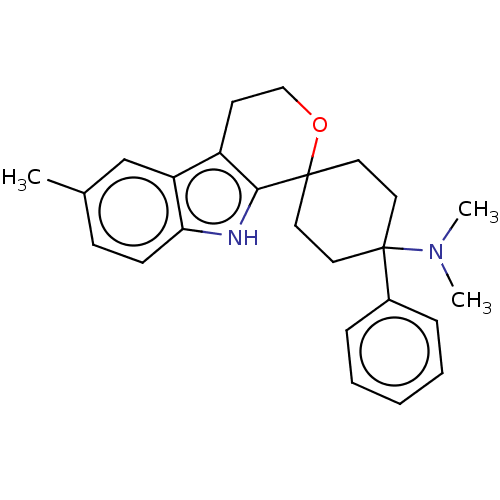 | Wt: 346.4 BDBM177930 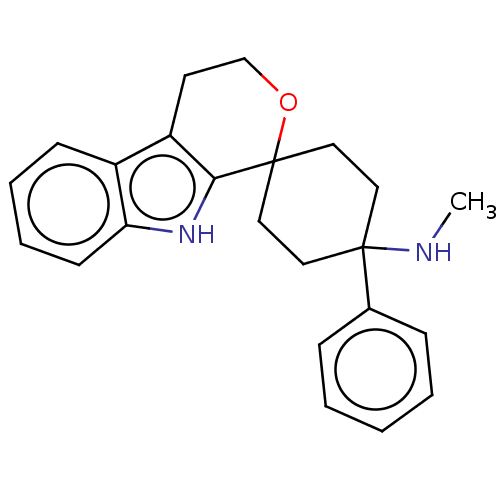 |
| Wt: 373.5 BDBM177931 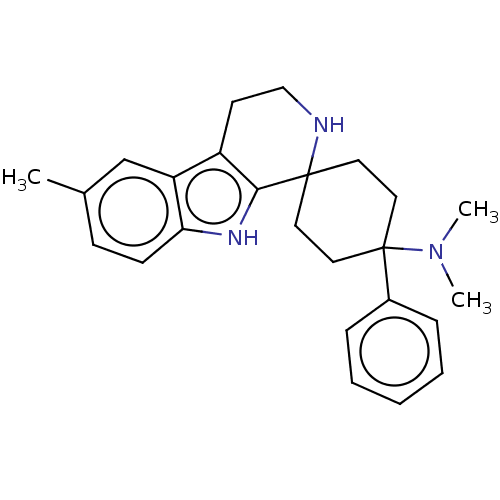 | Wt: 373.5 BDBM177935  | Wt: 364.4 BDBM177951  Purchase Purchase | Wt: 373.5 BDBM177955 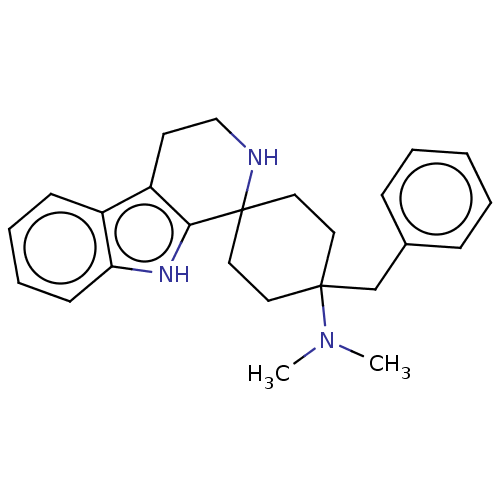 | Wt: 369.5 BDBM172408 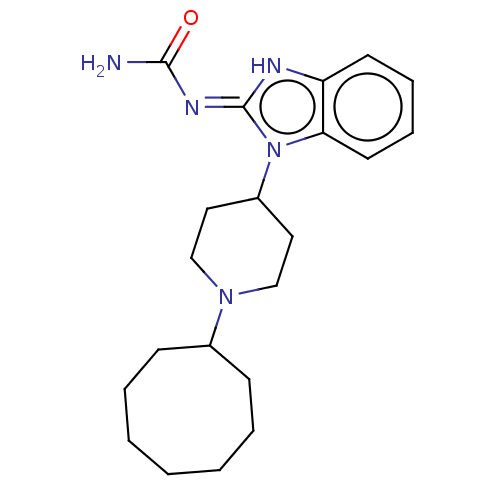 |
| Displayed 1 to 15 (of 439 total ) | Next | Last >> |
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM177911 (US9120797, 10 | US9120797, 9) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.260 | -54.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Gruenenthal GmbH US Patent | Assay Description The cyclohexane derivatives of the general formula I were investigated in a receptor binding assay with 3H-nociceptin/orphanin FQ with membranes from... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM177903 (US9120797, 1 | US9120797, 2 | US9120797, 3) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.300 | -54.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Gruenenthal GmbH US Patent | Assay Description The cyclohexane derivatives of the general formula I were investigated in a receptor binding assay with 3H-nociceptin/orphanin FQ with membranes from... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM177911 (US9120797, 10 | US9120797, 9) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.360 | -53.9 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Gruenenthal GmbH US Patent | Assay Description The receptor affinity for the human mu-opiate receptor was determined in a homogeneous batch in microtitre plates. For this, dilution series of the p... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM177935 (US9120797, 33) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | -53.1 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Gruenenthal GmbH US Patent | Assay Description The receptor affinity for the human mu-opiate receptor was determined in a homogeneous batch in microtitre plates. For this, dilution series of the p... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM177935 (US9120797, 33) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | -53.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Gruenenthal GmbH US Patent | Assay Description The cyclohexane derivatives of the general formula I were investigated in a receptor binding assay with 3H-nociceptin/orphanin FQ with membranes from... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM177955 (US9120797, 53) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.600 | -52.6 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Gruenenthal GmbH US Patent | Assay Description The receptor affinity for the human mu-opiate receptor was determined in a homogeneous batch in microtitre plates. For this, dilution series of the p... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM177903 (US9120797, 1 | US9120797, 2 | US9120797, 3) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.600 | -52.6 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Gruenenthal GmbH US Patent | Assay Description The receptor affinity for the human mu-opiate receptor was determined in a homogeneous batch in microtitre plates. For this, dilution series of the p... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM177903 (US9120797, 1 | US9120797, 2 | US9120797, 3) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.600 | -52.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Gruenenthal GmbH US Patent | Assay Description The cyclohexane derivatives of the general formula I were investigated in a receptor binding assay with 3H-nociceptin/orphanin FQ with membranes from... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM177955 (US9120797, 53) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.10 | -51.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Gruenenthal GmbH US Patent | Assay Description The cyclohexane derivatives of the general formula I were investigated in a receptor binding assay with 3H-nociceptin/orphanin FQ with membranes from... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM177930 (US9120797, 28) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.20 | -50.9 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Gruenenthal GmbH US Patent | Assay Description The receptor affinity for the human mu-opiate receptor was determined in a homogeneous batch in microtitre plates. For this, dilution series of the p... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM177916 (US9120797, 14 | US9120797, 15) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.20 | -50.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Gruenenthal GmbH US Patent | Assay Description The cyclohexane derivatives of the general formula I were investigated in a receptor binding assay with 3H-nociceptin/orphanin FQ with membranes from... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM177903 (US9120797, 1 | US9120797, 2 | US9120797, 3) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.30 | -50.7 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Gruenenthal GmbH US Patent | Assay Description The receptor affinity for the human mu-opiate receptor was determined in a homogeneous batch in microtitre plates. For this, dilution series of the p... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM177930 (US9120797, 28) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.40 | -50.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Gruenenthal GmbH US Patent | Assay Description The cyclohexane derivatives of the general formula I were investigated in a receptor binding assay with 3H-nociceptin/orphanin FQ with membranes from... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM177928 (US9120797, 26) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.70 | -48.9 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Gruenenthal GmbH US Patent | Assay Description The receptor affinity for the human mu-opiate receptor was determined in a homogeneous batch in microtitre plates. For this, dilution series of the p... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM177911 (US9120797, 10 | US9120797, 9) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.40 | -48.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Gruenenthal GmbH US Patent | Assay Description The cyclohexane derivatives of the general formula I were investigated in a receptor binding assay with 3H-nociceptin/orphanin FQ with membranes from... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM177911 (US9120797, 10 | US9120797, 9) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 4.5 | -47.6 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Gruenenthal GmbH US Patent | Assay Description The receptor affinity for the human mu-opiate receptor was determined in a homogeneous batch in microtitre plates. For this, dilution series of the p... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM177928 (US9120797, 26) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 4.5 | -47.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Gruenenthal GmbH US Patent | Assay Description The cyclohexane derivatives of the general formula I were investigated in a receptor binding assay with 3H-nociceptin/orphanin FQ with membranes from... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM177951 (US9120797, 49) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | 6.80 | -46.6 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Gruenenthal GmbH US Patent | Assay Description The receptor affinity for the human mu-opiate receptor was determined in a homogeneous batch in microtitre plates. For this, dilution series of the p... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM172408 (US9090618, ZA27 | US9598411, Ref. No. ZA27) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P. US Patent | Assay Description ORL-1 Receptor Binding Assay Procedures: Membranes from recombinant HEK-293 cells expressing the human opioid receptor-like receptor (ORL-1) (Recepto... | US Patent US9598411 (2017) BindingDB Entry DOI: 10.7270/Q2ZG6V9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM177951 (US9120797, 49) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | 10 | -45.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Gruenenthal GmbH US Patent | Assay Description The cyclohexane derivatives of the general formula I were investigated in a receptor binding assay with 3H-nociceptin/orphanin FQ with membranes from... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM177916 (US9120797, 14 | US9120797, 15) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 12 | -45.2 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Gruenenthal GmbH US Patent | Assay Description The receptor affinity for the human mu-opiate receptor was determined in a homogeneous batch in microtitre plates. For this, dilution series of the p... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM177931 (US9120797, 29) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 15 | -44.7 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Gruenenthal GmbH US Patent | Assay Description The receptor affinity for the human mu-opiate receptor was determined in a homogeneous batch in microtitre plates. For this, dilution series of the p... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM133985 (US8846929, 15) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 15.4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Purdue Pharma L.P.; Shionogi & Co., Ltd. US Patent | Assay Description Radioligand binding assays (screening and dose-displacement) used 0.1nM [3H]-nociceptin (NEN; 87.7 Cl/nimole) with 10-20 ug membrane protein in a fin... | US Patent US8846929 (2014) BindingDB Entry DOI: 10.7270/Q2G44P04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM133912 (US8846929, 88) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 20.8 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Purdue Pharma L.P.; Shionogi & Co., Ltd. US Patent | Assay Description Radioligand binding assays (screening and dose-displacement) used 0.1nM [3H]-nociceptin (NEN; 87.7 Cl/nimole) with 10-20 ug membrane protein in a fin... | US Patent US8846929 (2014) BindingDB Entry DOI: 10.7270/Q2G44P04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM177931 (US9120797, 29) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 32 | -42.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Gruenenthal GmbH US Patent | Assay Description The cyclohexane derivatives of the general formula I were investigated in a receptor binding assay with 3H-nociceptin/orphanin FQ with membranes from... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM172408 (US9090618, ZA27 | US9598411, Ref. No. ZA27) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 33.7 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Purdue Pharma L.P. US Patent | Assay Description Radioligand dose displacement assays used 0.4-0.8 nM [3H]-U69,593 (NEN; 40 Ci/mmole) with 10-20 μg membrane protein (recombinant kappa opioid re... | US Patent US9090618 (2015) BindingDB Entry DOI: 10.7270/Q2G73CGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM172408 (US9090618, ZA27 | US9598411, Ref. No. ZA27) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 33.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P. US Patent | Assay Description μ-Opioid Receptor Binding Assay Procedures: Radioligand binding assays were conducted using freshly thawed membranes expressing human μ-rec... | US Patent US9598411 (2017) BindingDB Entry DOI: 10.7270/Q2ZG6V9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM172408 (US9090618, ZA27 | US9598411, Ref. No. ZA27) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 36.7 | -42.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Purdue Pharma L.P. US Patent | Assay Description Radioligand binding assays (screening and dose-displacement) used 0.1 nM [3H]-nociceptin (NEN; 87.7 Ci/mmole) with 10-20 ug membrane protein in a fin... | US Patent US9090618 (2015) BindingDB Entry DOI: 10.7270/Q2G73CGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM172408 (US9090618, ZA27 | US9598411, Ref. No. ZA27) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 36.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P. US Patent | Assay Description δ-Opioid Receptor Binding Assay Procedures: Radioligand dose-displacement assays used 0.2 nM [3H]-Naltrindole (NEN; 33.0 Ci/mmole) with 10-20 &#... | US Patent US9598411 (2017) BindingDB Entry DOI: 10.7270/Q2ZG6V9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM177916 (US9120797, 14 | US9120797, 15) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 42 | -42.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Gruenenthal GmbH US Patent | Assay Description The cyclohexane derivatives of the general formula I were investigated in a receptor binding assay with 3H-nociceptin/orphanin FQ with membranes from... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM177916 (US9120797, 14 | US9120797, 15) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 58 | -41.3 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Gruenenthal GmbH US Patent | Assay Description The receptor affinity for the human mu-opiate receptor was determined in a homogeneous batch in microtitre plates. For this, dilution series of the p... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM133996 (US8846929, 86) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 83.5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Purdue Pharma L.P.; Shionogi & Co., Ltd. US Patent | Assay Description Radioligand dose displacement assays used 0.4-0.8nM [3H]-U69,593 (NEN; 40 Ci/mmole) with 10-20 ug membrane protein (recombinant kappa opioid receptor... | US Patent US8846929 (2014) BindingDB Entry DOI: 10.7270/Q2G44P04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM133995 (US8846929, 85) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 92.2 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Purdue Pharma L.P.; Shionogi & Co., Ltd. US Patent | Assay Description Radioligand binding assays (screening and dose-displacement) used 0.1nM [3H]-nociceptin (NEN; 87.7 Cl/nimole) with 10-20 ug membrane protein in a fin... | US Patent US8846929 (2014) BindingDB Entry DOI: 10.7270/Q2G44P04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM133985 (US8846929, 15) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 92.9 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Purdue Pharma L.P.; Shionogi & Co., Ltd. US Patent | Assay Description Radioligand dose displacement assays used 0.4-0.8nM [3H]-U69,593 (NEN; 40 Ci/mmole) with 10-20 ug membrane protein (recombinant kappa opioid receptor... | US Patent US8846929 (2014) BindingDB Entry DOI: 10.7270/Q2G44P04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM133996 (US8846929, 86) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 179 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Purdue Pharma L.P.; Shionogi & Co., Ltd. US Patent | Assay Description Radioligand binding assays (screening and dose-displacement) used 0.1nM [3H]-nociceptin (NEN; 87.7 Cl/nimole) with 10-20 ug membrane protein in a fin... | US Patent US8846929 (2014) BindingDB Entry DOI: 10.7270/Q2G44P04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM133995 (US8846929, 85) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 182 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Purdue Pharma L.P.; Shionogi & Co., Ltd. US Patent | Assay Description Radioligand dose displacement assays used 0.4-0.8nM [3H]-U69,593 (NEN; 40 Ci/mmole) with 10-20 ug membrane protein (recombinant kappa opioid receptor... | US Patent US8846929 (2014) BindingDB Entry DOI: 10.7270/Q2G44P04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM177903 (US9120797, 1 | US9120797, 2 | US9120797, 3) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 310 | -37.1 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Gruenenthal GmbH US Patent | Assay Description The receptor affinity for the human mu-opiate receptor was determined in a homogeneous batch in microtitre plates. For this, dilution series of the p... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM172408 (US9090618, ZA27 | US9598411, Ref. No. ZA27) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 339 | -36.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Purdue Pharma L.P. US Patent | Assay Description Radioligand binding assays were conducted using freshly thawed membranes expressing human mu-receptors (Perkin Elmer, Shelton, Conn.). Radioligand do... | US Patent US9090618 (2015) BindingDB Entry DOI: 10.7270/Q2G73CGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM172408 (US9090618, ZA27 | US9598411, Ref. No. ZA27) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 339 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P. US Patent | Assay Description κ-Opioid Receptor Binding Assay Procedures: Membranes from recombinant HEK-293 cells expressing the human kappa opioid receptor (kappa) (cloned ... | US Patent US9598411 (2017) BindingDB Entry DOI: 10.7270/Q2ZG6V9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM133912 (US8846929, 88) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 536 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Purdue Pharma L.P.; Shionogi & Co., Ltd. US Patent | Assay Description Radioligand dose displacement assays used 0.4-0.8nM [3H]-U69,593 (NEN; 40 Ci/mmole) with 10-20 ug membrane protein (recombinant kappa opioid receptor... | US Patent US8846929 (2014) BindingDB Entry DOI: 10.7270/Q2G44P04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM133985 (US8846929, 15) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Purdue Pharma L.P.; Shionogi & Co., Ltd. US Patent | Assay Description Radioligand dose-displacement binding assays for u-opioid receptors used 0.2nM[3H]-diprenorphine (NEN, Boston, Mass.), with 5-20mg membrane protein/w... | US Patent US8846929 (2014) BindingDB Entry DOI: 10.7270/Q2G44P04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM133912 (US8846929, 88) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Purdue Pharma L.P.; Shionogi & Co., Ltd. US Patent | Assay Description Radioligand dose-displacement binding assays for u-opioid receptors used 0.2nM[3H]-diprenorphine (NEN, Boston, Mass.), with 5-20mg membrane protein/w... | US Patent US8846929 (2014) BindingDB Entry DOI: 10.7270/Q2G44P04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM18576 (US8846929, 365) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Purdue Pharma L.P.; Shionogi & Co., Ltd. US Patent | Assay Description Radioligand binding assays (screening and dose-displacement) used 0.1nM [3H]-nociceptin (NEN; 87.7 Cl/nimole) with 10-20 ug membrane protein in a fin... | US Patent US8846929 (2014) BindingDB Entry DOI: 10.7270/Q2G44P04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM133995 (US8846929, 85) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 4.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Purdue Pharma L.P.; Shionogi & Co., Ltd. US Patent | Assay Description Radioligand dose-displacement binding assays for u-opioid receptors used 0.2nM[3H]-diprenorphine (NEN, Boston, Mass.), with 5-20mg membrane protein/w... | US Patent US8846929 (2014) BindingDB Entry DOI: 10.7270/Q2G44P04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM133996 (US8846929, 86) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 6.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Purdue Pharma L.P.; Shionogi & Co., Ltd. US Patent | Assay Description Radioligand dose-displacement binding assays for u-opioid receptors used 0.2nM[3H]-diprenorphine (NEN, Boston, Mass.), with 5-20mg membrane protein/w... | US Patent US8846929 (2014) BindingDB Entry DOI: 10.7270/Q2G44P04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM133996 (US8846929, 86) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 9.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Purdue Pharma L.P.; Shionogi & Co., Ltd. US Patent | Assay Description Radioligand dose-displacement assays used 0.2nM [3H]-Naltrindole (NEN; 33.0 Ci/mmole) with 10-20ug membrane protein (recombinant delta opioid recepto... | US Patent US8846929 (2014) BindingDB Entry DOI: 10.7270/Q2G44P04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM172408 (US9090618, ZA27 | US9598411, Ref. No. ZA27) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Purdue Pharma L.P. US Patent | Assay Description Radioligand dose-displacement assays used 0.2 nM [3H]-Naltrindole (NEN; 33.0 Ci/mmole) with 10-20 μg membrane protein (recombinant delta opioid ... | US Patent US9090618 (2015) BindingDB Entry DOI: 10.7270/Q2G73CGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM133912 (US8846929, 88) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Purdue Pharma L.P.; Shionogi & Co., Ltd. US Patent | Assay Description Radioligand dose-displacement assays used 0.2nM [3H]-Naltrindole (NEN; 33.0 Ci/mmole) with 10-20ug membrane protein (recombinant delta opioid recepto... | US Patent US8846929 (2014) BindingDB Entry DOI: 10.7270/Q2G44P04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM133995 (US8846929, 85) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Purdue Pharma L.P.; Shionogi & Co., Ltd. US Patent | Assay Description Radioligand dose-displacement assays used 0.2nM [3H]-Naltrindole (NEN; 33.0 Ci/mmole) with 10-20ug membrane protein (recombinant delta opioid recepto... | US Patent US8846929 (2014) BindingDB Entry DOI: 10.7270/Q2G44P04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM133985 (US8846929, 15) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Purdue Pharma L.P.; Shionogi & Co., Ltd. US Patent | Assay Description Radioligand dose-displacement assays used 0.2nM [3H]-Naltrindole (NEN; 33.0 Ci/mmole) with 10-20ug membrane protein (recombinant delta opioid recepto... | US Patent US8846929 (2014) BindingDB Entry DOI: 10.7270/Q2G44P04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||