 Found 43 hits with Last Name = 'drouillard' and Initial = 'a'
Found 43 hits with Last Name = 'drouillard' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50559603

(CHEMBL4787458)Show SMILES Cc1cc(OC2CCC3(CCCC3)CC2)c(CN2CCC(CC2)C(O)=O)c2ccccc12 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to recombinant human S1P5 receptor expressed in Chem-1 cell membrane by 33P-SIP binding assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50559628
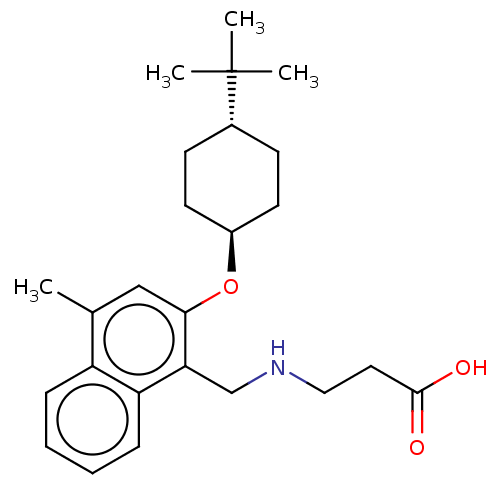
(CHEMBL4798593)Show SMILES Cc1cc(O[C@H]2CC[C@@H](CC2)C(C)(C)C)c(CNCCC(O)=O)c2ccccc12 |r,wU:5.4,wD:8.11,(15.03,-5.2,;15.04,-6.74,;13.71,-7.51,;13.71,-9.06,;12.37,-9.83,;11.04,-9.06,;9.71,-9.82,;8.38,-9.04,;8.38,-7.5,;9.72,-6.74,;11.05,-7.51,;7.05,-6.72,;7.06,-5.18,;5.72,-7.49,;5.71,-5.95,;15.04,-9.83,;15.04,-11.37,;16.38,-12.14,;17.71,-11.36,;19.05,-12.13,;20.38,-11.36,;21.72,-12.12,;20.38,-9.82,;16.37,-9.05,;17.71,-9.82,;19.04,-9.04,;19.04,-7.49,;17.7,-6.73,;16.37,-7.51,)| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to recombinant human S1P5 receptor expressed in Chem-1 cell membrane by 33P-SIP binding assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50559616
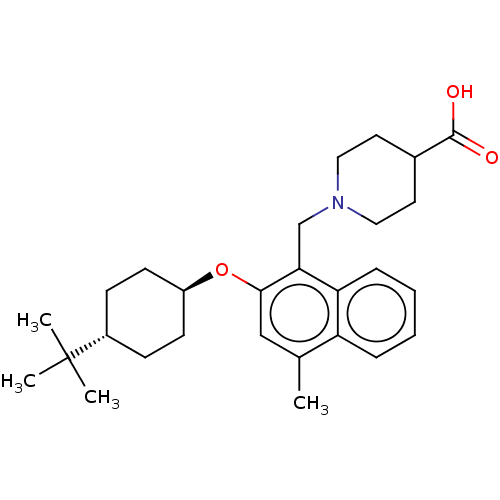
(CHEMBL4748198)Show SMILES Cc1cc(O[C@H]2CC[C@@H](CC2)C(C)(C)C)c(CN2CCC(CC2)C(O)=O)c2ccccc12 |r,wU:8.11,wD:5.4,(21.06,-7.5,;21.06,-9.04,;19.74,-9.81,;19.73,-11.36,;18.4,-12.13,;17.07,-11.36,;17.07,-9.81,;15.75,-9.04,;14.41,-9.8,;14.41,-11.34,;15.74,-12.12,;13.08,-9.02,;13.09,-7.48,;11.74,-9.79,;11.74,-8.25,;21.07,-12.13,;21.07,-13.67,;22.41,-14.44,;22.4,-15.98,;23.73,-16.74,;25.07,-15.98,;25.07,-14.43,;23.73,-13.66,;26.4,-16.75,;27.74,-15.98,;26.4,-18.29,;22.4,-11.35,;23.74,-12.12,;25.07,-11.34,;25.06,-9.79,;23.72,-9.03,;22.4,-9.81,)| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to recombinant human S1P5 receptor expressed in Chem-1 cell membrane by 33P-SIP binding assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50559616

(CHEMBL4748198)Show SMILES Cc1cc(O[C@H]2CC[C@@H](CC2)C(C)(C)C)c(CN2CCC(CC2)C(O)=O)c2ccccc12 |r,wU:8.11,wD:5.4,(21.06,-7.5,;21.06,-9.04,;19.74,-9.81,;19.73,-11.36,;18.4,-12.13,;17.07,-11.36,;17.07,-9.81,;15.75,-9.04,;14.41,-9.8,;14.41,-11.34,;15.74,-12.12,;13.08,-9.02,;13.09,-7.48,;11.74,-9.79,;11.74,-8.25,;21.07,-12.13,;21.07,-13.67,;22.41,-14.44,;22.4,-15.98,;23.73,-16.74,;25.07,-15.98,;25.07,-14.43,;23.73,-13.66,;26.4,-16.75,;27.74,-15.98,;26.4,-18.29,;22.4,-11.35,;23.74,-12.12,;25.07,-11.34,;25.06,-9.79,;23.72,-9.03,;22.4,-9.81,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to recombinant human S1P1 receptor expressed in Chem-1 cell membrane by 33P-SIP binding assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50559628

(CHEMBL4798593)Show SMILES Cc1cc(O[C@H]2CC[C@@H](CC2)C(C)(C)C)c(CNCCC(O)=O)c2ccccc12 |r,wU:5.4,wD:8.11,(15.03,-5.2,;15.04,-6.74,;13.71,-7.51,;13.71,-9.06,;12.37,-9.83,;11.04,-9.06,;9.71,-9.82,;8.38,-9.04,;8.38,-7.5,;9.72,-6.74,;11.05,-7.51,;7.05,-6.72,;7.06,-5.18,;5.72,-7.49,;5.71,-5.95,;15.04,-9.83,;15.04,-11.37,;16.38,-12.14,;17.71,-11.36,;19.05,-12.13,;20.38,-11.36,;21.72,-12.12,;20.38,-9.82,;16.37,-9.05,;17.71,-9.82,;19.04,-9.04,;19.04,-7.49,;17.7,-6.73,;16.37,-7.51,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to recombinant human S1P1 receptor expressed in Chem-1 cell membrane by 33P-SIP binding assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50559616

(CHEMBL4748198)Show SMILES Cc1cc(O[C@H]2CC[C@@H](CC2)C(C)(C)C)c(CN2CCC(CC2)C(O)=O)c2ccccc12 |r,wU:8.11,wD:5.4,(21.06,-7.5,;21.06,-9.04,;19.74,-9.81,;19.73,-11.36,;18.4,-12.13,;17.07,-11.36,;17.07,-9.81,;15.75,-9.04,;14.41,-9.8,;14.41,-11.34,;15.74,-12.12,;13.08,-9.02,;13.09,-7.48,;11.74,-9.79,;11.74,-8.25,;21.07,-12.13,;21.07,-13.67,;22.41,-14.44,;22.4,-15.98,;23.73,-16.74,;25.07,-15.98,;25.07,-14.43,;23.73,-13.66,;26.4,-16.75,;27.74,-15.98,;26.4,-18.29,;22.4,-11.35,;23.74,-12.12,;25.07,-11.34,;25.06,-9.79,;23.72,-9.03,;22.4,-9.81,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50559616

(CHEMBL4748198)Show SMILES Cc1cc(O[C@H]2CC[C@@H](CC2)C(C)(C)C)c(CN2CCC(CC2)C(O)=O)c2ccccc12 |r,wU:8.11,wD:5.4,(21.06,-7.5,;21.06,-9.04,;19.74,-9.81,;19.73,-11.36,;18.4,-12.13,;17.07,-11.36,;17.07,-9.81,;15.75,-9.04,;14.41,-9.8,;14.41,-11.34,;15.74,-12.12,;13.08,-9.02,;13.09,-7.48,;11.74,-9.79,;11.74,-8.25,;21.07,-12.13,;21.07,-13.67,;22.41,-14.44,;22.4,-15.98,;23.73,-16.74,;25.07,-15.98,;25.07,-14.43,;23.73,-13.66,;26.4,-16.75,;27.74,-15.98,;26.4,-18.29,;22.4,-11.35,;23.74,-12.12,;25.07,-11.34,;25.06,-9.79,;23.72,-9.03,;22.4,-9.81,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50559616

(CHEMBL4748198)Show SMILES Cc1cc(O[C@H]2CC[C@@H](CC2)C(C)(C)C)c(CN2CCC(CC2)C(O)=O)c2ccccc12 |r,wU:8.11,wD:5.4,(21.06,-7.5,;21.06,-9.04,;19.74,-9.81,;19.73,-11.36,;18.4,-12.13,;17.07,-11.36,;17.07,-9.81,;15.75,-9.04,;14.41,-9.8,;14.41,-11.34,;15.74,-12.12,;13.08,-9.02,;13.09,-7.48,;11.74,-9.79,;11.74,-8.25,;21.07,-12.13,;21.07,-13.67,;22.41,-14.44,;22.4,-15.98,;23.73,-16.74,;25.07,-15.98,;25.07,-14.43,;23.73,-13.66,;26.4,-16.75,;27.74,-15.98,;26.4,-18.29,;22.4,-11.35,;23.74,-12.12,;25.07,-11.34,;25.06,-9.79,;23.72,-9.03,;22.4,-9.81,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50559616

(CHEMBL4748198)Show SMILES Cc1cc(O[C@H]2CC[C@@H](CC2)C(C)(C)C)c(CN2CCC(CC2)C(O)=O)c2ccccc12 |r,wU:8.11,wD:5.4,(21.06,-7.5,;21.06,-9.04,;19.74,-9.81,;19.73,-11.36,;18.4,-12.13,;17.07,-11.36,;17.07,-9.81,;15.75,-9.04,;14.41,-9.8,;14.41,-11.34,;15.74,-12.12,;13.08,-9.02,;13.09,-7.48,;11.74,-9.79,;11.74,-8.25,;21.07,-12.13,;21.07,-13.67,;22.41,-14.44,;22.4,-15.98,;23.73,-16.74,;25.07,-15.98,;25.07,-14.43,;23.73,-13.66,;26.4,-16.75,;27.74,-15.98,;26.4,-18.29,;22.4,-11.35,;23.74,-12.12,;25.07,-11.34,;25.06,-9.79,;23.72,-9.03,;22.4,-9.81,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50559616

(CHEMBL4748198)Show SMILES Cc1cc(O[C@H]2CC[C@@H](CC2)C(C)(C)C)c(CN2CCC(CC2)C(O)=O)c2ccccc12 |r,wU:8.11,wD:5.4,(21.06,-7.5,;21.06,-9.04,;19.74,-9.81,;19.73,-11.36,;18.4,-12.13,;17.07,-11.36,;17.07,-9.81,;15.75,-9.04,;14.41,-9.8,;14.41,-11.34,;15.74,-12.12,;13.08,-9.02,;13.09,-7.48,;11.74,-9.79,;11.74,-8.25,;21.07,-12.13,;21.07,-13.67,;22.41,-14.44,;22.4,-15.98,;23.73,-16.74,;25.07,-15.98,;25.07,-14.43,;23.73,-13.66,;26.4,-16.75,;27.74,-15.98,;26.4,-18.29,;22.4,-11.35,;23.74,-12.12,;25.07,-11.34,;25.06,-9.79,;23.72,-9.03,;22.4,-9.81,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50559613

(CHEMBL4758438)Show SMILES CC(C)(C)[C@H]1CC[C@@H](CC1)Oc1cc(I)c2ccccc2c1CN1CCC(CC1)C(O)=O |r,wU:4.3,wD:7.10,(12.78,-7.84,;12.78,-9.38,;11.44,-10.15,;11.43,-8.61,;14.11,-10.16,;15.45,-9.4,;16.77,-10.17,;16.76,-11.71,;15.44,-12.48,;14.1,-11.7,;18.1,-12.49,;19.43,-11.72,;19.43,-10.17,;20.76,-9.4,;20.76,-7.86,;22.1,-10.17,;23.42,-9.39,;24.76,-10.15,;24.77,-11.7,;23.43,-12.48,;22.1,-11.71,;20.76,-12.49,;20.77,-14.03,;22.1,-14.79,;22.1,-16.33,;23.43,-17.1,;24.77,-16.33,;24.77,-14.79,;23.43,-14.02,;26.1,-17.11,;27.43,-16.34,;26.1,-18.65,)| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 165 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at recombinant human S1P5 receptor expressed in Chem-1 cells assessed as EC80 S1P-induced calcium flux measured for 180 secs by F... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50559614
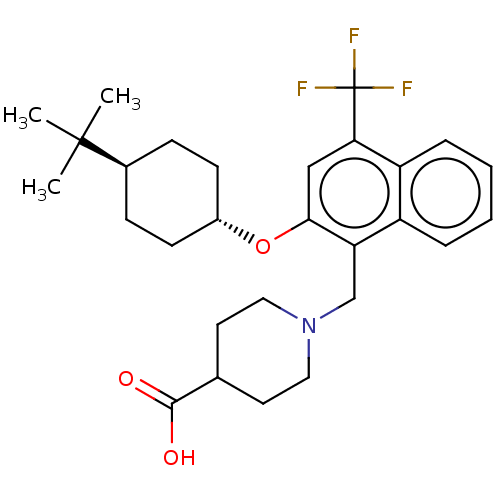
(CHEMBL4793349)Show SMILES CC(C)(C)[C@H]1CC[C@@H](CC1)Oc1cc(c2ccccc2c1CN1CCC(CC1)C(O)=O)C(F)(F)F |r,wU:4.3,wD:7.10,(13.43,-8.3,;13.42,-9.84,;12.09,-10.61,;12.08,-9.07,;14.75,-10.62,;16.09,-9.86,;17.42,-10.63,;17.41,-12.17,;16.08,-12.94,;14.75,-12.16,;18.74,-12.94,;20.08,-12.18,;20.08,-10.63,;21.41,-9.86,;22.74,-10.62,;24.07,-9.85,;25.4,-10.61,;25.41,-12.16,;24.08,-12.94,;22.74,-12.17,;21.41,-12.95,;21.41,-14.49,;22.75,-15.25,;22.75,-16.79,;24.08,-17.56,;25.41,-16.79,;25.41,-15.25,;24.08,-14.48,;26.75,-17.57,;28.08,-16.8,;26.74,-19.11,;21.4,-8.32,;20.07,-7.56,;22.73,-7.55,;21.39,-6.77,)| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at recombinant human S1P5 receptor expressed in Chem-1 cells assessed as EC80 S1P-induced calcium flux measured for 180 secs by F... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50559615
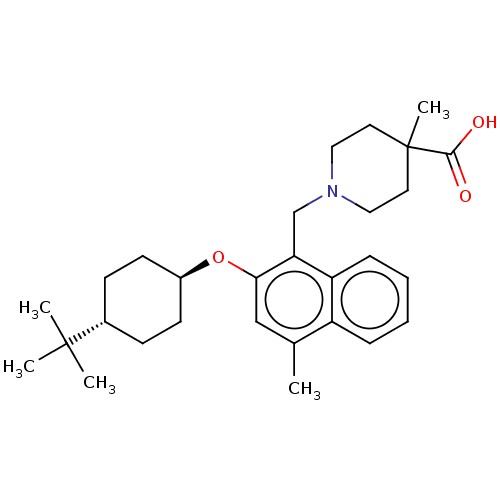
(CHEMBL4765011)Show SMILES Cc1cc(O[C@H]2CC[C@@H](CC2)C(C)(C)C)c(CN2CCC(C)(CC2)C(O)=O)c2ccccc12 |r,wU:8.11,wD:5.4,(21.06,-7.5,;21.07,-9.04,;19.74,-9.82,;19.73,-11.36,;18.4,-12.13,;17.07,-11.36,;17.08,-9.81,;15.75,-9.04,;14.41,-9.8,;14.41,-11.34,;15.74,-12.12,;13.08,-9.03,;13.09,-7.49,;11.74,-9.79,;11.74,-8.25,;21.07,-12.13,;21.07,-13.67,;22.41,-14.44,;22.41,-15.98,;23.73,-16.74,;25.07,-15.98,;25.06,-17.52,;25.07,-14.44,;23.74,-13.66,;26.4,-16.75,;27.74,-15.98,;26.4,-18.29,;22.4,-11.35,;23.74,-12.12,;25.07,-11.34,;25.06,-9.8,;23.73,-9.04,;22.4,-9.81,)| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.81E+3 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at recombinant human S1P5 receptor expressed in Chem-1 cells assessed as EC80 S1P-induced calcium flux measured for 180 secs by F... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50559616

(CHEMBL4748198)Show SMILES Cc1cc(O[C@H]2CC[C@@H](CC2)C(C)(C)C)c(CN2CCC(CC2)C(O)=O)c2ccccc12 |r,wU:8.11,wD:5.4,(21.06,-7.5,;21.06,-9.04,;19.74,-9.81,;19.73,-11.36,;18.4,-12.13,;17.07,-11.36,;17.07,-9.81,;15.75,-9.04,;14.41,-9.8,;14.41,-11.34,;15.74,-12.12,;13.08,-9.02,;13.09,-7.48,;11.74,-9.79,;11.74,-8.25,;21.07,-12.13,;21.07,-13.67,;22.41,-14.44,;22.4,-15.98,;23.73,-16.74,;25.07,-15.98,;25.07,-14.43,;23.73,-13.66,;26.4,-16.75,;27.74,-15.98,;26.4,-18.29,;22.4,-11.35,;23.74,-12.12,;25.07,-11.34,;25.06,-9.79,;23.72,-9.03,;22.4,-9.81,)| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at recombinant human S1P5 receptor expressed in Chem-1 cells assessed as EC80 S1P-induced calcium flux measured for 180 secs by F... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50559617
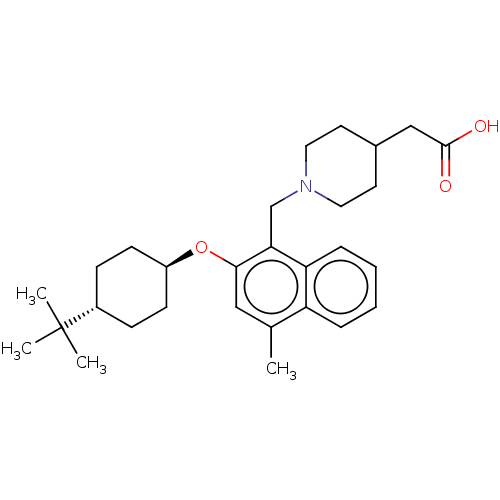
(CHEMBL4791545)Show SMILES Cc1cc(O[C@H]2CC[C@@H](CC2)C(C)(C)C)c(CN2CCC(CC(O)=O)CC2)c2ccccc12 |r,wU:8.11,wD:5.4,(18.32,-4.12,;18.33,-5.66,;17,-6.43,;17,-7.98,;15.66,-8.75,;14.33,-7.97,;14.34,-6.43,;13.01,-5.66,;11.67,-6.42,;11.67,-7.96,;13,-8.74,;10.34,-5.64,;10.35,-4.1,;9.01,-6.41,;9,-4.87,;18.33,-8.75,;18.33,-10.29,;19.67,-11.05,;19.67,-12.59,;21,-13.36,;22.33,-12.59,;23.67,-13.37,;23.66,-14.91,;22.33,-15.67,;25,-15.68,;22.33,-11.05,;21,-10.28,;19.66,-7.97,;21,-8.74,;22.33,-7.96,;22.33,-6.41,;20.99,-5.65,;19.66,-6.43,)| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at recombinant human S1P5 receptor expressed in Chem-1 cells assessed as EC80 S1P-induced calcium flux measured for 180 secs by F... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50559618
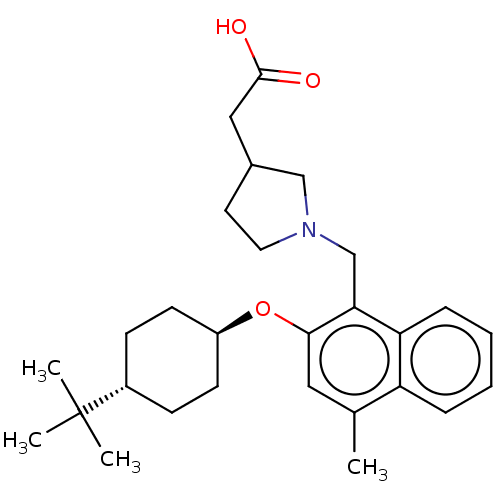
(CHEMBL4744455)Show SMILES Cc1cc(O[C@H]2CC[C@@H](CC2)C(C)(C)C)c(CN2CCC(CC(O)=O)C2)c2ccccc12 |r,wU:8.11,wD:5.4,(11.28,-6.95,;11.28,-8.49,;9.95,-9.26,;9.95,-10.81,;8.62,-11.58,;7.28,-10.81,;7.29,-9.26,;5.97,-8.49,;4.63,-9.25,;4.62,-10.79,;5.96,-11.57,;3.3,-8.47,;3.3,-6.93,;1.96,-9.24,;1.95,-7.7,;11.28,-11.58,;11.29,-13.12,;12.62,-13.88,;12.79,-15.42,;14.3,-15.73,;15.06,-14.4,;16.6,-14.23,;17.5,-15.48,;19.03,-15.31,;16.88,-16.88,;14.03,-13.25,;12.62,-10.8,;13.95,-11.57,;15.29,-10.79,;15.28,-9.24,;13.94,-8.48,;12.62,-9.26,)| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at recombinant human S1P5 receptor expressed in Chem-1 cells assessed as EC80 S1P-induced calcium flux measured for 180 secs by F... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50559619

(CHEMBL4798181)Show SMILES Cc1cc(O[C@H]2CC[C@@H](CC2)C(C)(C)C)c(CN2CCC(C2)C(O)=O)c2ccccc12 |r,wU:8.11,wD:5.4,(11.56,-5.73,;11.56,-7.27,;10.23,-8.04,;10.23,-9.59,;8.9,-10.35,;7.56,-9.58,;7.57,-8.04,;6.25,-7.27,;4.91,-8.03,;4.9,-9.57,;6.24,-10.35,;3.58,-7.25,;3.58,-5.71,;2.24,-8.02,;2.23,-6.48,;11.56,-10.36,;11.57,-11.9,;12.9,-12.66,;13.07,-14.19,;14.58,-14.51,;15.34,-13.17,;14.31,-12.03,;16.87,-13.01,;17.78,-14.25,;17.5,-11.6,;12.9,-9.58,;14.23,-10.35,;15.57,-9.57,;15.56,-8.02,;14.22,-7.26,;12.9,-8.03,)| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.960 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at recombinant human S1P5 receptor expressed in Chem-1 cells assessed as EC80 S1P-induced calcium flux measured for 180 secs by F... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50559620
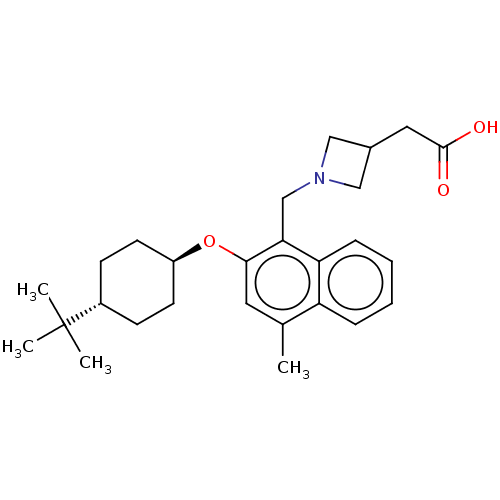
(CHEMBL4744077)Show SMILES Cc1cc(O[C@H]2CC[C@@H](CC2)C(C)(C)C)c(CN2CC(CC(O)=O)C2)c2ccccc12 |r,wU:8.11,wD:5.4,(11.68,-4.8,;11.69,-6.34,;10.36,-7.11,;10.36,-8.65,;9.02,-9.42,;7.69,-8.65,;7.7,-7.1,;6.37,-6.33,;5.03,-7.09,;5.03,-8.64,;6.36,-9.42,;3.7,-6.32,;3.71,-4.78,;2.36,-7.08,;2.36,-5.55,;11.69,-9.42,;11.69,-10.96,;13.03,-11.73,;13.44,-13.21,;14.92,-12.81,;16.26,-13.58,;16.26,-15.12,;17.6,-15.88,;14.93,-15.89,;14.52,-11.32,;13.02,-8.65,;14.36,-9.41,;15.69,-8.64,;15.68,-7.09,;14.35,-6.33,;13.02,-7.1,)| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at recombinant human S1P5 receptor expressed in Chem-1 cells assessed as EC80 S1P-induced calcium flux measured for 180 secs by F... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50559621

(CHEMBL4796939)Show SMILES Cc1cc(O[C@H]2CC[C@@H](CC2)C(C)(C)C)c(CN2CC(C2)C(O)=O)c2ccccc12 |r,wU:8.11,wD:5.4,(11.68,-4.8,;11.69,-6.34,;10.36,-7.11,;10.36,-8.65,;9.02,-9.42,;7.69,-8.65,;7.7,-7.1,;6.37,-6.33,;5.03,-7.09,;5.03,-8.64,;6.36,-9.42,;3.7,-6.32,;3.71,-4.78,;2.36,-7.08,;2.36,-5.55,;11.69,-9.42,;11.69,-10.96,;13.03,-11.73,;13.44,-13.21,;14.92,-12.81,;14.52,-11.32,;16.26,-13.58,;16.26,-15.12,;17.59,-12.8,;13.02,-8.65,;14.36,-9.41,;15.69,-8.64,;15.68,-7.09,;14.35,-6.33,;13.02,-7.1,)| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at recombinant human S1P5 receptor expressed in Chem-1 cells assessed as EC80 S1P-induced calcium flux measured for 180 secs by F... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50559622
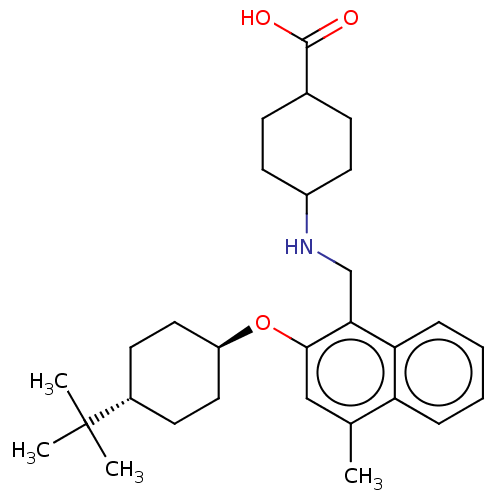
(CHEMBL4784199)Show SMILES Cc1cc(O[C@H]2CC[C@@H](CC2)C(C)(C)C)c(CNC2CCC(CC2)C(O)=O)c2ccccc12 |r,wU:8.11,wD:5.4,(14.18,-2.77,;14.18,-4.31,;12.85,-5.08,;12.85,-6.62,;11.52,-7.39,;10.18,-6.62,;10.19,-5.07,;8.87,-4.3,;7.53,-5.06,;7.52,-6.61,;8.86,-7.39,;6.2,-4.29,;6.21,-2.75,;4.86,-5.05,;4.85,-3.52,;14.19,-7.39,;14.19,-8.93,;15.52,-9.7,;15.53,-11.24,;14.19,-12.01,;14.19,-13.54,;15.52,-14.31,;16.86,-13.54,;16.86,-12,;15.52,-15.85,;16.85,-16.63,;14.19,-16.62,;15.52,-6.62,;16.85,-7.38,;18.19,-6.61,;18.18,-5.06,;16.84,-4.3,;15.52,-5.07,)| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at recombinant human S1P5 receptor expressed in Chem-1 cells assessed as EC80 S1P-induced calcium flux measured for 180 secs by F... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50559623
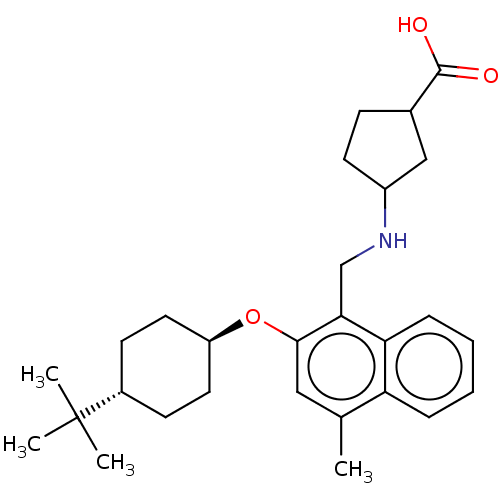
(CHEMBL4747262)Show SMILES Cc1cc(O[C@H]2CC[C@@H](CC2)C(C)(C)C)c(CNC2CCC(C2)C(O)=O)c2ccccc12 |r,wU:8.11,wD:5.4,(14.87,-3.29,;14.87,-4.83,;13.55,-5.6,;13.54,-7.14,;12.21,-7.91,;10.88,-7.14,;10.89,-5.59,;9.56,-4.82,;8.22,-5.59,;8.22,-7.13,;9.55,-7.91,;6.89,-4.81,;6.9,-3.27,;5.55,-5.57,;5.55,-4.04,;14.88,-7.91,;14.88,-9.45,;16.22,-10.22,;16.22,-11.76,;17.47,-12.66,;17,-14.13,;15.46,-14.13,;14.98,-12.67,;14.56,-15.38,;13.03,-15.22,;15.19,-16.79,;16.21,-7.14,;17.55,-7.9,;18.88,-7.13,;18.87,-5.58,;17.54,-4.82,;16.21,-5.59,)| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at recombinant human S1P5 receptor expressed in Chem-1 cells assessed as EC80 S1P-induced calcium flux measured for 180 secs by F... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50559624

(CHEMBL4754965)Show SMILES Cc1cc(O[C@H]2CC[C@@H](CC2)C(C)(C)C)c(CNC2CC(C2)C(O)=O)c2ccccc12 |r,wU:8.11,wD:5.4,(15.22,-4.91,;15.22,-6.45,;13.9,-7.23,;13.89,-8.77,;12.56,-9.54,;11.23,-8.77,;11.24,-7.22,;9.91,-6.45,;8.57,-7.21,;8.57,-8.75,;9.9,-9.53,;7.24,-6.44,;7.25,-4.9,;5.9,-7.2,;5.9,-5.66,;15.23,-9.54,;15.23,-11.08,;16.57,-11.85,;16.57,-13.39,;15.48,-14.47,;16.57,-15.56,;17.66,-14.47,;16.58,-17.1,;15.25,-17.87,;17.91,-17.87,;16.56,-8.76,;17.9,-9.53,;19.23,-8.75,;19.22,-7.21,;17.89,-6.45,;16.56,-7.22,)| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0630 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at recombinant human S1P5 receptor expressed in Chem-1 cells assessed as EC80 S1P-induced calcium flux measured for 180 secs by F... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50559625
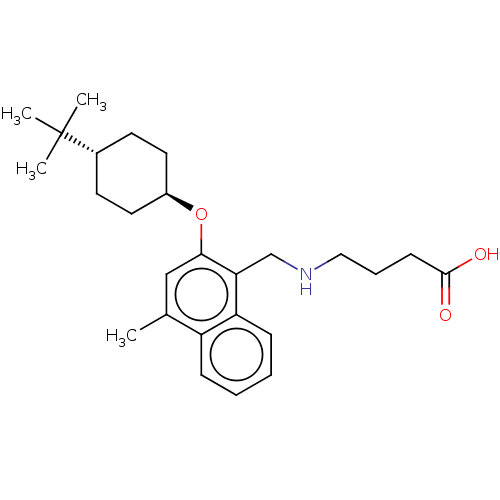
(CHEMBL4778095)Show SMILES Cc1cc(O[C@H]2CC[C@@H](CC2)C(C)(C)C)c(CNCCCC(O)=O)c2ccccc12 |r,wU:8.11,wD:5.4,(11.95,-5.46,;11.95,-7,;10.62,-7.77,;10.62,-9.31,;9.29,-10.08,;7.95,-9.31,;7.96,-7.76,;6.64,-6.99,;5.3,-7.76,;5.29,-9.3,;6.63,-10.08,;3.97,-6.98,;3.97,-5.44,;2.63,-7.74,;2.62,-6.21,;11.95,-10.08,;11.96,-11.62,;13.29,-12.39,;13.3,-13.93,;14.63,-14.7,;14.64,-16.24,;15.97,-17.01,;15.97,-18.55,;17.3,-16.23,;13.29,-9.31,;14.62,-10.07,;15.96,-9.3,;15.95,-7.75,;14.61,-6.99,;13.29,-7.76,)| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at recombinant human S1P5 receptor expressed in Chem-1 cells assessed as EC80 S1P-induced calcium flux measured for 180 secs by F... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50559626
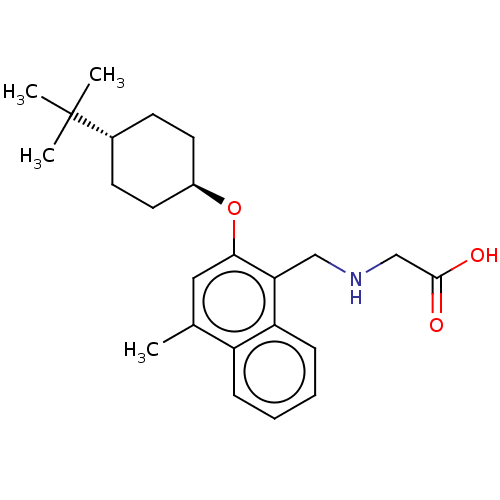
(CHEMBL4798888)Show SMILES Cc1cc(O[C@H]2CC[C@@H](CC2)C(C)(C)C)c(CNCC(O)=O)c2ccccc12 |r,wU:8.11,wD:5.4,(14.63,-4.87,;14.63,-6.41,;13.3,-7.18,;13.3,-8.72,;11.97,-9.49,;10.63,-8.72,;10.64,-7.17,;9.32,-6.4,;7.98,-7.16,;7.97,-8.71,;9.31,-9.49,;6.65,-6.39,;6.66,-4.85,;5.31,-7.15,;5.3,-5.62,;14.64,-9.49,;14.64,-11.03,;15.98,-11.8,;15.98,-13.34,;17.31,-14.11,;17.32,-15.65,;18.65,-13.33,;15.97,-8.71,;17.3,-9.48,;18.64,-8.71,;18.63,-7.16,;17.29,-6.4,;15.97,-7.17,)| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 267 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at recombinant human S1P5 receptor expressed in Chem-1 cells assessed as EC80 S1P-induced calcium flux measured for 180 secs by F... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50559627

(CHEMBL4760451)Show SMILES CC(C)(C)[C@H]1CC[C@@H](CC1)Oc1cc(I)c2ccccc2c1CNCCC(O)=O |r,wU:7.10,wD:4.3,(5.96,-6.93,;5.95,-8.47,;4.61,-9.24,;4.6,-7.7,;7.28,-9.25,;7.27,-10.79,;8.61,-11.57,;9.94,-10.8,;9.94,-9.26,;8.62,-8.49,;11.27,-11.58,;12.6,-10.81,;12.6,-9.26,;13.93,-8.49,;13.93,-6.95,;15.27,-9.26,;16.59,-8.48,;17.93,-9.24,;17.94,-10.79,;16.6,-11.57,;15.27,-10.8,;13.94,-11.58,;13.94,-13.12,;15.28,-13.88,;16.61,-13.11,;17.94,-13.88,;19.27,-13.11,;20.61,-13.87,;19.27,-11.57,)| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at recombinant human S1P5 receptor expressed in Chem-1 cells assessed as EC80 S1P-induced calcium flux measured for 180 secs by F... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50559628

(CHEMBL4798593)Show SMILES Cc1cc(O[C@H]2CC[C@@H](CC2)C(C)(C)C)c(CNCCC(O)=O)c2ccccc12 |r,wU:5.4,wD:8.11,(15.03,-5.2,;15.04,-6.74,;13.71,-7.51,;13.71,-9.06,;12.37,-9.83,;11.04,-9.06,;9.71,-9.82,;8.38,-9.04,;8.38,-7.5,;9.72,-6.74,;11.05,-7.51,;7.05,-6.72,;7.06,-5.18,;5.72,-7.49,;5.71,-5.95,;15.04,-9.83,;15.04,-11.37,;16.38,-12.14,;17.71,-11.36,;19.05,-12.13,;20.38,-11.36,;21.72,-12.12,;20.38,-9.82,;16.37,-9.05,;17.71,-9.82,;19.04,-9.04,;19.04,-7.49,;17.7,-6.73,;16.37,-7.51,)| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at recombinant human S1P5 receptor expressed in Chem-1 cells assessed as EC80 S1P-induced calcium flux measured for 180 secs by F... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50559629
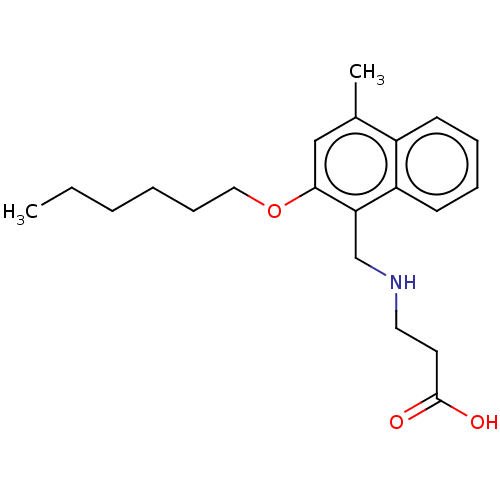
(CHEMBL4751484) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at recombinant human S1P5 receptor expressed in Chem-1 cells assessed as EC80 S1P-induced calcium flux measured for 180 secs by F... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50559630
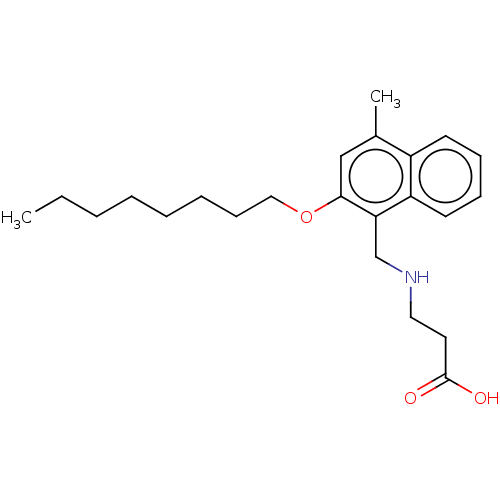
(CHEMBL4742052) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 406 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at recombinant human S1P5 receptor expressed in Chem-1 cells assessed as EC80 S1P-induced calcium flux measured for 180 secs by F... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM23165

(CHEMBL366208 | FTY720-phosphate, (S)-2 | [(2S)-2-a...)Show SMILES CCCCCCCCc1ccc(CC[C@](N)(CO)COP(O)(O)=O)cc1 |r| Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at recombinant human S1P1 receptor expressed in Chem-1 cells assessed as calcium flux measured for 180 secs by FLIPR assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sphingosine 1-phosphate receptor 2
(Homo sapiens (Human)) | BDBM23165

(CHEMBL366208 | FTY720-phosphate, (S)-2 | [(2S)-2-a...)Show SMILES CCCCCCCCc1ccc(CC[C@](N)(CO)COP(O)(O)=O)cc1 |r| Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24)/t19-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >5 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at recombinant human S1P2 receptor expressed in Chem-1 cells assessed as calcium flux measured for 180 secs by FLIPR assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM23165

(CHEMBL366208 | FTY720-phosphate, (S)-2 | [(2S)-2-a...)Show SMILES CCCCCCCCc1ccc(CC[C@](N)(CO)COP(O)(O)=O)cc1 |r| Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24)/t19-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 27 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at recombinant human S1P3 receptor expressed in Chem-1 cells assessed as calcium flux measured for 180 secs by FLIPR assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sphingosine 1-phosphate receptor 4
(Homo sapiens (Human)) | BDBM23165

(CHEMBL366208 | FTY720-phosphate, (S)-2 | [(2S)-2-a...)Show SMILES CCCCCCCCc1ccc(CC[C@](N)(CO)COP(O)(O)=O)cc1 |r| Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24)/t19-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 22 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at recombinant human S1P4 receptor expressed in Chem-1 cells assessed as calcium flux measured for 180 secs by FLIPR assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50559612

(CHEMBL4740175)Show SMILES CC(C)(C)[C@H]1CC[C@@H](CC1)Oc1cc(Cl)c2ccccc2c1CN1CCC(CC1)C(O)=O |r,wU:4.3,wD:7.10,(13.41,-5.68,;13.4,-7.22,;12.06,-7.98,;12.05,-6.45,;14.73,-8,;16.07,-7.23,;17.39,-8,;17.38,-9.55,;16.06,-10.32,;14.72,-9.54,;18.72,-10.32,;20.05,-9.55,;20.05,-8.01,;21.38,-7.24,;21.38,-5.7,;22.72,-8,;24.04,-7.23,;25.38,-7.99,;25.39,-9.54,;24.05,-10.31,;22.72,-9.55,;21.39,-10.32,;21.39,-11.86,;22.72,-12.63,;22.72,-14.17,;24.05,-14.94,;25.39,-14.17,;25.39,-12.63,;24.05,-11.85,;26.72,-14.94,;28.05,-14.18,;26.72,-16.48,)| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 36 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at recombinant human S1P5 receptor expressed in Chem-1 cells assessed as EC80 S1P-induced calcium flux measured for 180 secs by F... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50559611

(CHEMBL4762836)Show SMILES CC(C)(C)[C@H]1CC[C@@H](CC1)Oc1ccc2ccccc2c1CN1CCC(CC1)C(O)=O |r,wU:4.3,wD:7.10,(13.41,-5.68,;13.4,-7.22,;12.06,-7.98,;12.05,-6.45,;14.73,-8,;16.07,-7.23,;17.39,-8,;17.38,-9.55,;16.06,-10.32,;14.72,-9.54,;18.72,-10.32,;20.05,-9.55,;20.05,-8.01,;21.38,-7.24,;22.72,-8,;24.04,-7.23,;25.38,-7.99,;25.39,-9.54,;24.05,-10.31,;22.72,-9.55,;21.39,-10.32,;21.39,-11.86,;22.72,-12.63,;22.72,-14.17,;24.05,-14.94,;25.39,-14.17,;25.39,-12.63,;24.05,-11.85,;26.72,-14.94,;28.05,-14.18,;26.72,-16.48,)| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 602 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at recombinant human S1P5 receptor expressed in Chem-1 cells assessed as EC80 S1P-induced calcium flux measured for 180 secs by F... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50559610

(CHEMBL4779029)Show SMILES CCC(C)(C)[C@H]1CC[C@@H](CC1)Oc1cc(C)c2ccccc2c1CN1CCC(CC1)C(O)=O |r,wU:8.11,wD:5.4,(11.06,-4.53,;11.06,-6.07,;12.41,-6.84,;12.41,-5.3,;11.07,-7.6,;13.74,-7.62,;13.73,-9.16,;15.06,-9.94,;16.39,-9.17,;16.4,-7.62,;15.07,-6.85,;17.72,-9.94,;19.06,-9.17,;19.06,-7.63,;20.39,-6.86,;20.38,-5.32,;21.72,-7.62,;23.05,-6.85,;24.39,-7.61,;24.4,-9.16,;23.06,-9.93,;21.73,-9.17,;20.39,-9.94,;20.4,-11.48,;21.73,-12.25,;21.73,-13.79,;23.06,-14.56,;24.39,-13.79,;24.39,-12.25,;23.06,-11.47,;25.73,-14.56,;27.06,-13.8,;25.72,-16.1,)| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 67 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at recombinant human S1P5 receptor expressed in Chem-1 cells assessed as EC80 S1P-induced calcium flux measured for 180 secs by F... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50559609
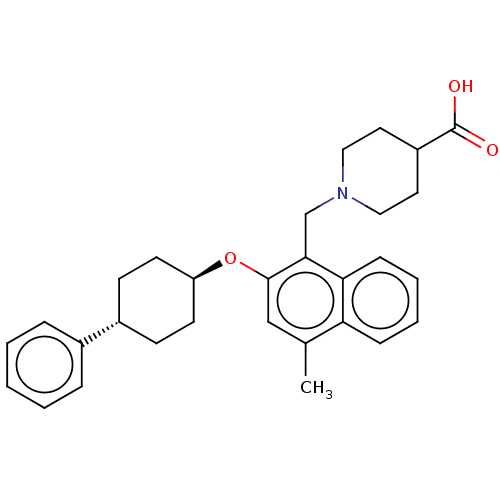
(CHEMBL4795566)Show SMILES Cc1cc(O[C@H]2CC[C@@H](CC2)c2ccccc2)c(CN2CCC(CC2)C(O)=O)c2ccccc12 |r,wU:5.4,wD:8.11,(20.27,-4.38,;20.27,-5.92,;18.94,-6.69,;18.94,-8.23,;17.61,-9,;16.27,-8.23,;14.95,-9,;13.61,-8.22,;13.62,-6.67,;14.96,-5.91,;16.28,-6.68,;12.29,-5.9,;12.3,-4.36,;10.97,-3.58,;9.63,-4.35,;9.63,-5.9,;10.96,-6.66,;20.28,-9,;20.28,-10.54,;21.61,-11.31,;21.61,-12.85,;22.94,-13.62,;24.28,-12.85,;24.28,-11.31,;22.94,-10.53,;25.61,-13.62,;26.95,-12.85,;25.61,-15.16,;21.61,-8.23,;22.94,-8.99,;24.28,-8.22,;24.27,-6.67,;22.93,-5.91,;21.61,-6.68,)| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at recombinant human S1P5 receptor expressed in Chem-1 cells assessed as EC80 S1P-induced calcium flux measured for 180 secs by F... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50559608
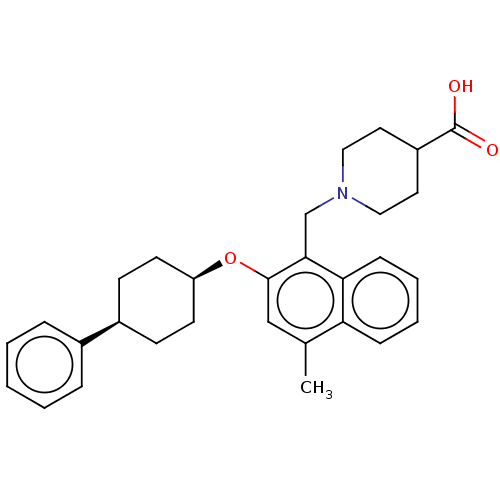
(CHEMBL4787014)Show SMILES Cc1cc(O[C@H]2CC[C@H](CC2)c2ccccc2)c(CN2CCC(CC2)C(O)=O)c2ccccc12 |r,wU:5.4,8.11,(20.79,-5.32,;20.79,-6.86,;19.46,-7.63,;19.46,-9.17,;18.13,-9.94,;16.8,-9.17,;15.47,-9.94,;14.13,-9.16,;14.14,-7.62,;15.48,-6.85,;16.8,-7.62,;12.81,-6.84,;12.82,-5.3,;11.49,-4.52,;10.15,-5.29,;10.15,-6.84,;11.48,-7.61,;20.8,-9.94,;20.8,-11.48,;22.14,-12.25,;22.13,-13.79,;23.46,-14.56,;24.8,-13.79,;24.8,-12.25,;23.46,-11.47,;26.13,-14.56,;27.47,-13.8,;26.13,-16.1,;22.13,-9.17,;23.47,-9.93,;24.8,-9.16,;24.79,-7.61,;23.45,-6.85,;22.13,-7.62,)| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.54E+3 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at recombinant human S1P5 receptor expressed in Chem-1 cells assessed as EC80 S1P-induced calcium flux measured for 180 secs by F... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50559607

(CHEMBL4764896)Show SMILES CC[C@H]1CC[C@H](CC1)Oc1cc(C)c2ccccc2c1CN1CCC(CC1)C(O)=O |r,wU:5.8,2.1,(11.48,-7.6,;12.81,-6.84,;14.14,-7.61,;15.48,-6.85,;16.8,-7.62,;16.79,-9.17,;15.47,-9.94,;14.13,-9.16,;18.13,-9.94,;19.46,-9.17,;19.46,-7.63,;20.79,-6.86,;20.79,-5.32,;22.13,-7.62,;23.45,-6.85,;24.79,-7.61,;24.8,-9.16,;23.46,-9.93,;22.13,-9.17,;20.79,-9.94,;20.8,-11.48,;22.13,-12.25,;22.13,-13.79,;23.46,-14.56,;24.8,-13.79,;24.8,-12.25,;23.46,-11.47,;26.13,-14.56,;27.46,-13.79,;26.13,-16.1,)| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.24E+3 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at recombinant human S1P5 receptor expressed in Chem-1 cells assessed as EC80 S1P-induced calcium flux measured for 180 secs by F... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50559606
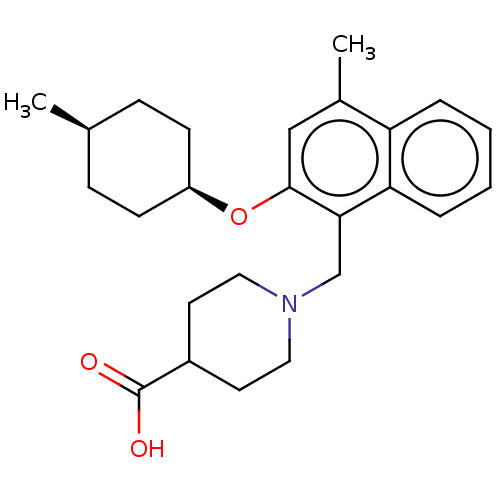
(CHEMBL4793467)Show SMILES C[C@H]1CC[C@H](CC1)Oc1cc(C)c2ccccc2c1CN1CCC(CC1)C(O)=O |r,wU:4.7,1.0,(11.94,-5.79,;13.27,-6.57,;14.61,-5.8,;15.93,-6.57,;15.92,-8.12,;14.6,-8.89,;13.26,-8.11,;17.26,-8.89,;18.59,-8.12,;18.59,-6.58,;19.92,-5.81,;19.92,-4.27,;21.26,-6.57,;22.58,-5.8,;23.92,-6.56,;23.93,-8.11,;22.59,-8.88,;21.26,-8.12,;19.93,-8.89,;19.93,-10.43,;21.26,-11.2,;21.26,-12.74,;22.59,-13.51,;23.93,-12.74,;23.93,-11.2,;22.59,-10.42,;25.26,-13.51,;26.59,-12.74,;25.26,-15.05,)| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at recombinant human S1P5 receptor expressed in Chem-1 cells assessed as EC80 S1P-induced calcium flux measured for 180 secs by F... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50559605
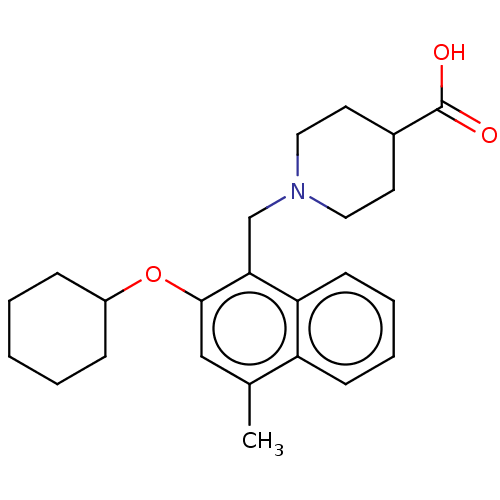
(CHEMBL4778961)Show SMILES Cc1cc(OC2CCCCC2)c(CN2CCC(CC2)C(O)=O)c2ccccc12 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 236 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at recombinant human S1P5 receptor expressed in Chem-1 cells assessed as EC80 S1P-induced calcium flux measured for 180 secs by F... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM23165

(CHEMBL366208 | FTY720-phosphate, (S)-2 | [(2S)-2-a...)Show SMILES CCCCCCCCc1ccc(CC[C@](N)(CO)COP(O)(O)=O)cc1 |r| Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24)/t19-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at recombinant human S1P5 receptor expressed in Chem-1 cells assessed as calcium flux measured for 180 secs by FLIPR assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50559603

(CHEMBL4787458)Show SMILES Cc1cc(OC2CCC3(CCCC3)CC2)c(CN2CCC(CC2)C(O)=O)c2ccccc12 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at recombinant human S1P5 receptor expressed in Chem-1 cells assessed as EC80 S1P-induced calcium flux measured for 180 secs by F... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50559604
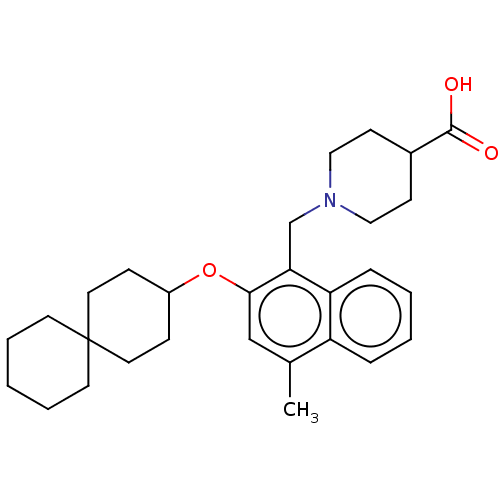
(CHEMBL4757160)Show SMILES Cc1cc(OC2CCC3(CCCCC3)CC2)c(CN2CCC(CC2)C(O)=O)c2ccccc12 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at recombinant human S1P5 receptor expressed in Chem-1 cells assessed as EC80 S1P-induced calcium flux measured for 180 secs by F... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data