

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase (Homo sapiens (Human)) | BDBM50005711 (CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of HDAC in human HeLa cell nuclear extract using BML-KI104 Fluor de Lys as substrate by fluorescence-based assay | J Nat Prod 78: 2867-79 (2015) Article DOI: 10.1021/acs.jnatprod.5b00700 BindingDB Entry DOI: 10.7270/Q2PG1VR7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Gujrat Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate pretreated for 10 mins followed by substrate addition measured after 20 m... | Bioorg Med Chem 26: 3696-3706 (2018) Article DOI: 10.1016/j.bmc.2018.05.050 BindingDB Entry DOI: 10.7270/Q2V69N32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Gujrat Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine iodide as substrate pretreated for 10 mins followed by substrate addition measured after 20 m... | Bioorg Med Chem 26: 3696-3706 (2018) Article DOI: 10.1016/j.bmc.2018.05.050 BindingDB Entry DOI: 10.7270/Q2V69N32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50140172 (CHEBI:3962 | CHEMBL140 | Curcumin | US9409845, Tab...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as reduction of PGE2 formation from PGH2 preincubated for 15 mins by... | J Nat Prod 78: 2867-79 (2015) Article DOI: 10.1021/acs.jnatprod.5b00700 BindingDB Entry DOI: 10.7270/Q2PG1VR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50500813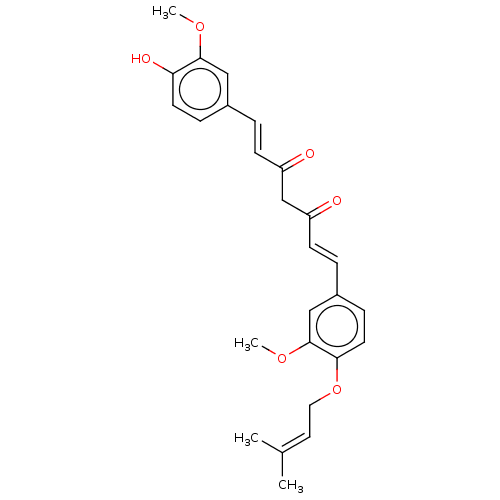 (CHEMBL1087690) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as reduction of PGE2 formation from PGH2 preincubated for 15 mins by... | J Nat Prod 78: 2867-79 (2015) Article DOI: 10.1021/acs.jnatprod.5b00700 BindingDB Entry DOI: 10.7270/Q2PG1VR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50500810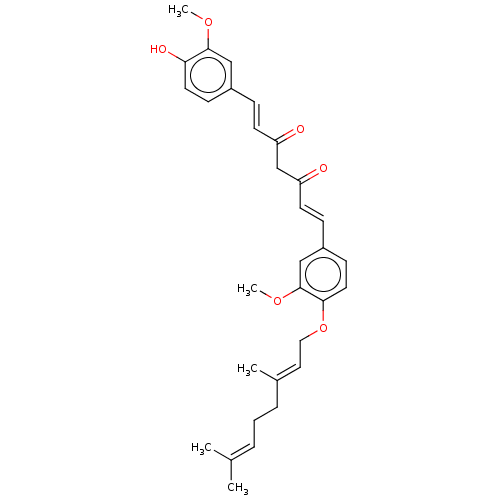 (CHEMBL3758791) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as reduction of PGE2 formation from PGH2 preincubated for 15 mins by... | J Nat Prod 78: 2867-79 (2015) Article DOI: 10.1021/acs.jnatprod.5b00700 BindingDB Entry DOI: 10.7270/Q2PG1VR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50500817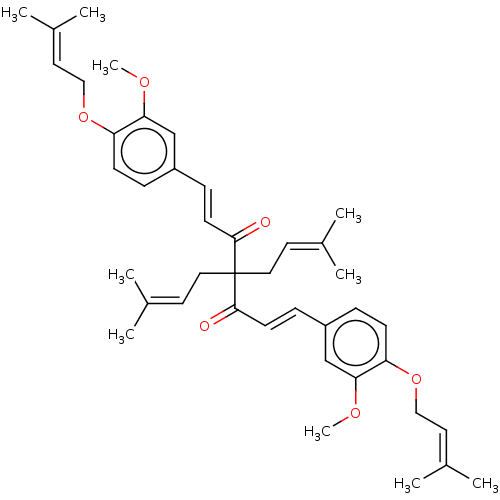 (CHEMBL3758432) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as reduction of PGE2 formation from PGH2 preincubated for 15 mins by... | J Nat Prod 78: 2867-79 (2015) Article DOI: 10.1021/acs.jnatprod.5b00700 BindingDB Entry DOI: 10.7270/Q2PG1VR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50500816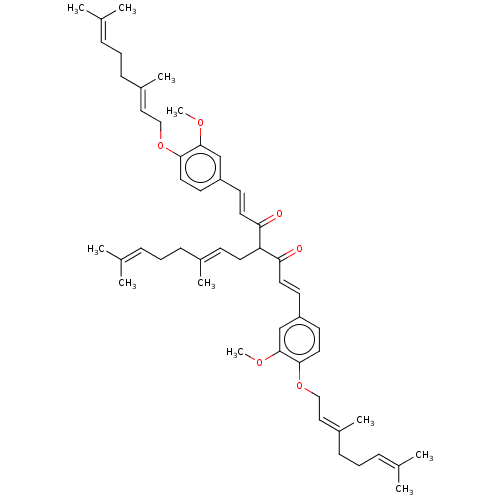 (CHEMBL3758656) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as reduction of PGE2 formation from PGH2 preincubated for 15 mins by... | J Nat Prod 78: 2867-79 (2015) Article DOI: 10.1021/acs.jnatprod.5b00700 BindingDB Entry DOI: 10.7270/Q2PG1VR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50500809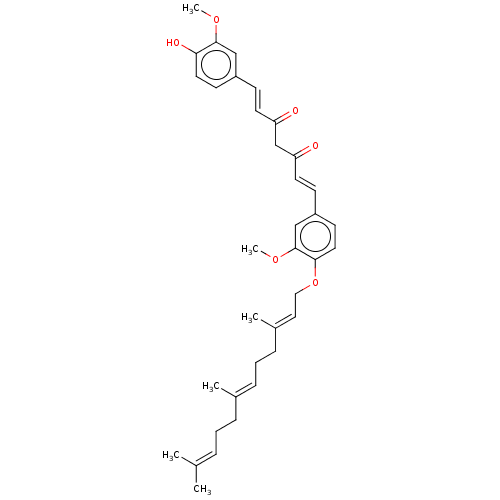 (CHEMBL3759529) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as reduction of PGE2 formation from PGH2 preincubated for 15 mins by... | J Nat Prod 78: 2867-79 (2015) Article DOI: 10.1021/acs.jnatprod.5b00700 BindingDB Entry DOI: 10.7270/Q2PG1VR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50500815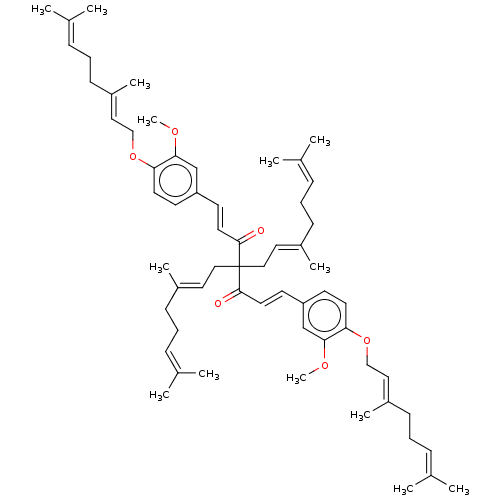 (CHEMBL3759699) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as reduction of PGE2 formation from PGH2 preincubated for 15 mins by... | J Nat Prod 78: 2867-79 (2015) Article DOI: 10.1021/acs.jnatprod.5b00700 BindingDB Entry DOI: 10.7270/Q2PG1VR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50500812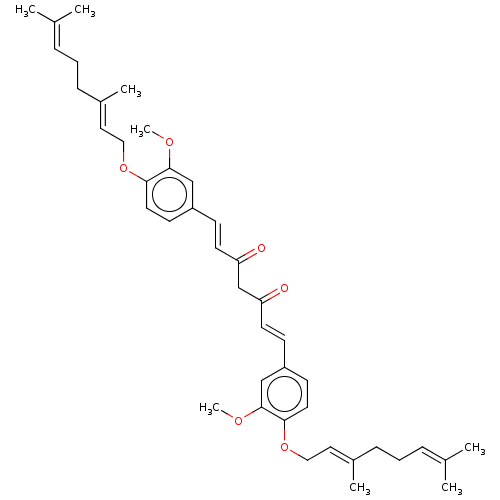 (CHEMBL3758253) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as reduction of PGE2 formation from PGH2 preincubated for 15 mins by... | J Nat Prod 78: 2867-79 (2015) Article DOI: 10.1021/acs.jnatprod.5b00700 BindingDB Entry DOI: 10.7270/Q2PG1VR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50500814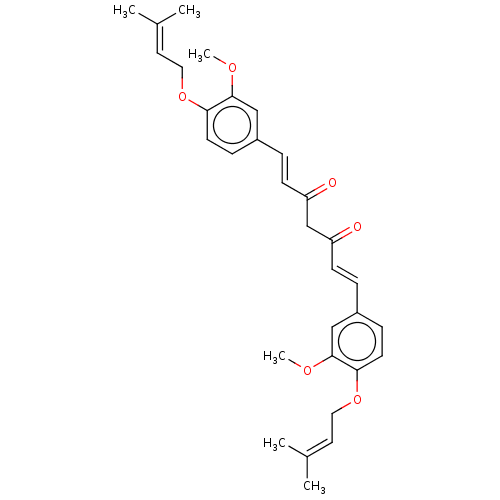 (CHEMBL1087807) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as reduction of PGE2 formation from PGH2 preincubated for 15 mins by... | J Nat Prod 78: 2867-79 (2015) Article DOI: 10.1021/acs.jnatprod.5b00700 BindingDB Entry DOI: 10.7270/Q2PG1VR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50500811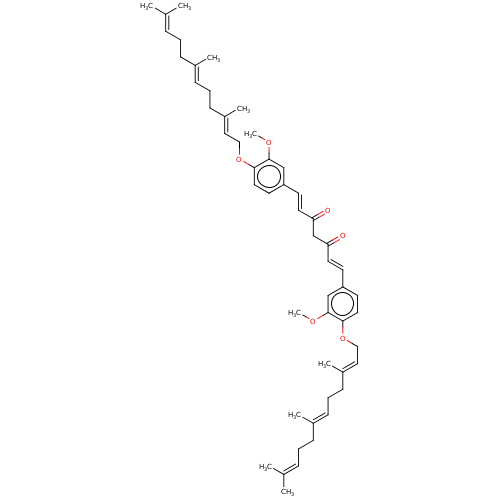 (CHEMBL3759749) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as reduction of PGE2 formation from PGH2 preincubated for 15 mins by... | J Nat Prod 78: 2867-79 (2015) Article DOI: 10.1021/acs.jnatprod.5b00700 BindingDB Entry DOI: 10.7270/Q2PG1VR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50500808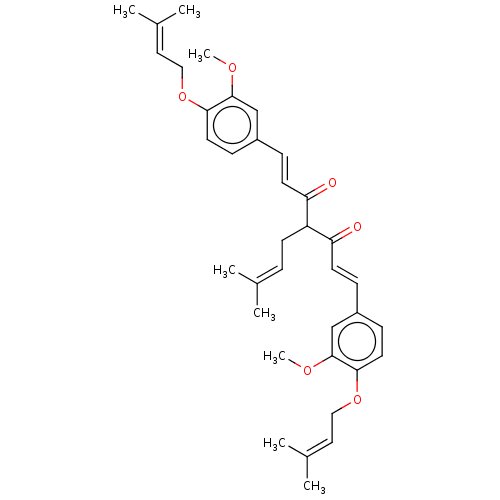 (CHEMBL3758528) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as reduction of PGE2 formation from PGH2 preincubated for 15 mins by... | J Nat Prod 78: 2867-79 (2015) Article DOI: 10.1021/acs.jnatprod.5b00700 BindingDB Entry DOI: 10.7270/Q2PG1VR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50276068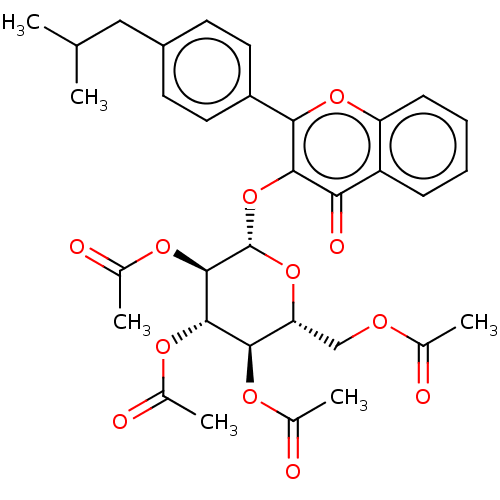 (CHEMBL4127173) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Gujrat Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate pretreated for 10 mins followed by substrate addition measured after 20 m... | Bioorg Med Chem 26: 3696-3706 (2018) Article DOI: 10.1016/j.bmc.2018.05.050 BindingDB Entry DOI: 10.7270/Q2V69N32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50276069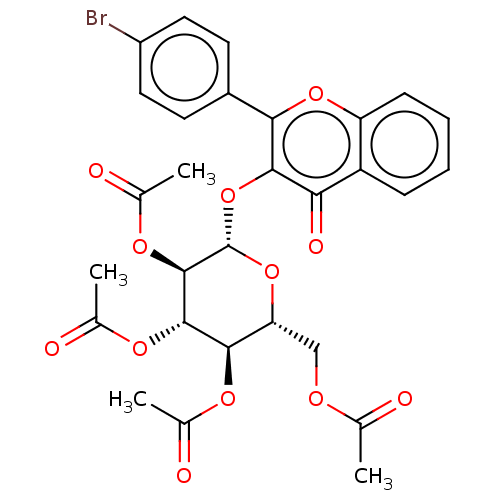 (CHEMBL4128561) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Gujrat Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine iodide as substrate pretreated for 10 mins followed by substrate addition measured after 20 m... | Bioorg Med Chem 26: 3696-3706 (2018) Article DOI: 10.1016/j.bmc.2018.05.050 BindingDB Entry DOI: 10.7270/Q2V69N32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50276068 (CHEMBL4127173) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Gujrat Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine iodide as substrate pretreated for 10 mins followed by substrate addition measured after 20 m... | Bioorg Med Chem 26: 3696-3706 (2018) Article DOI: 10.1016/j.bmc.2018.05.050 BindingDB Entry DOI: 10.7270/Q2V69N32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50276069 (CHEMBL4128561) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Gujrat Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate pretreated for 10 mins followed by substrate addition measured after 20 m... | Bioorg Med Chem 26: 3696-3706 (2018) Article DOI: 10.1016/j.bmc.2018.05.050 BindingDB Entry DOI: 10.7270/Q2V69N32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50276061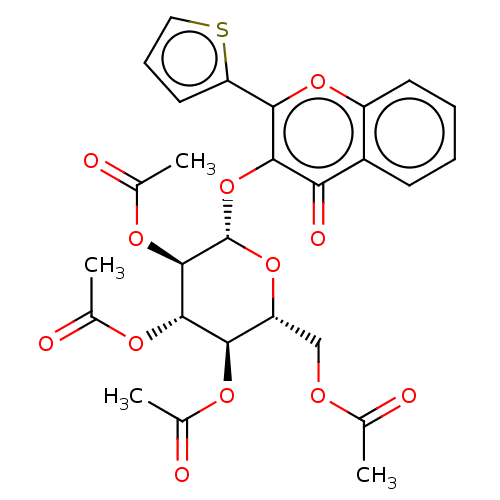 (CHEMBL4127253) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Gujrat Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate pretreated for 10 mins followed by substrate addition measured after 20 m... | Bioorg Med Chem 26: 3696-3706 (2018) Article DOI: 10.1016/j.bmc.2018.05.050 BindingDB Entry DOI: 10.7270/Q2V69N32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50276067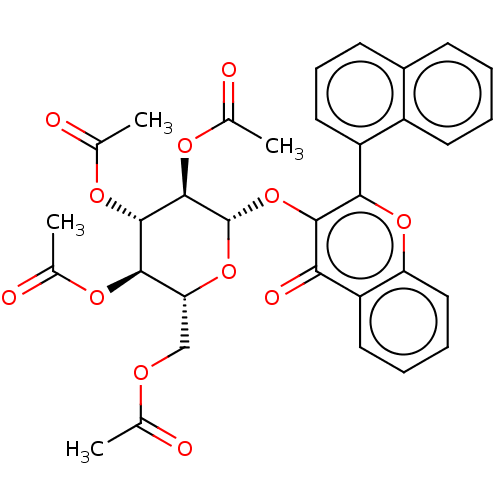 (CHEMBL4129776) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.87E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Gujrat Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine iodide as substrate pretreated for 10 mins followed by substrate addition measured after 20 m... | Bioorg Med Chem 26: 3696-3706 (2018) Article DOI: 10.1016/j.bmc.2018.05.050 BindingDB Entry DOI: 10.7270/Q2V69N32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50276059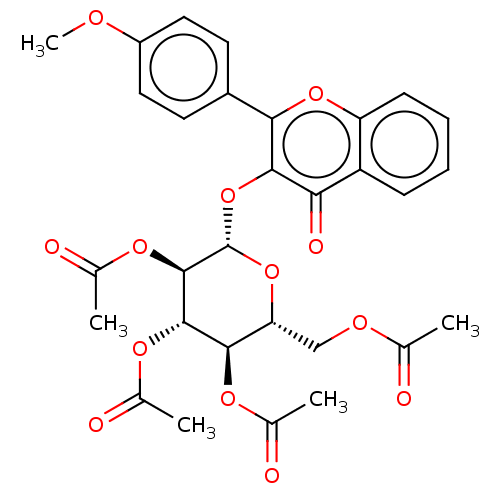 (CHEMBL4126201) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Gujrat Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate pretreated for 10 mins followed by substrate addition measured after 20 m... | Bioorg Med Chem 26: 3696-3706 (2018) Article DOI: 10.1016/j.bmc.2018.05.050 BindingDB Entry DOI: 10.7270/Q2V69N32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50276070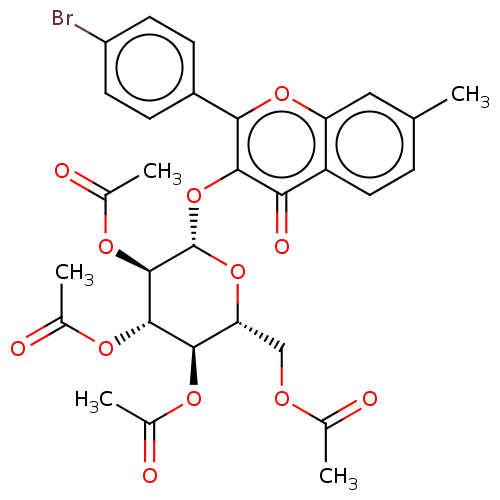 (CHEMBL4127574) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Gujrat Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate pretreated for 10 mins followed by substrate addition measured after 20 m... | Bioorg Med Chem 26: 3696-3706 (2018) Article DOI: 10.1016/j.bmc.2018.05.050 BindingDB Entry DOI: 10.7270/Q2V69N32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50276061 (CHEMBL4127253) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Gujrat Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine iodide as substrate pretreated for 10 mins followed by substrate addition measured after 20 m... | Bioorg Med Chem 26: 3696-3706 (2018) Article DOI: 10.1016/j.bmc.2018.05.050 BindingDB Entry DOI: 10.7270/Q2V69N32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50276059 (CHEMBL4126201) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Gujrat Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine iodide as substrate pretreated for 10 mins followed by substrate addition measured after 20 m... | Bioorg Med Chem 26: 3696-3706 (2018) Article DOI: 10.1016/j.bmc.2018.05.050 BindingDB Entry DOI: 10.7270/Q2V69N32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50276062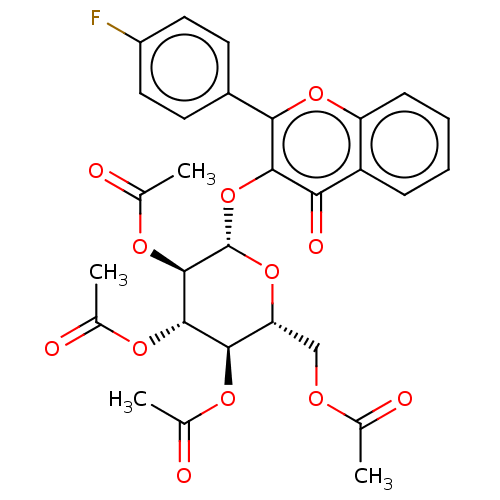 (CHEMBL4129855) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Gujrat Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate pretreated for 10 mins followed by substrate addition measured after 20 m... | Bioorg Med Chem 26: 3696-3706 (2018) Article DOI: 10.1016/j.bmc.2018.05.050 BindingDB Entry DOI: 10.7270/Q2V69N32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50276070 (CHEMBL4127574) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Gujrat Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine iodide as substrate pretreated for 10 mins followed by substrate addition measured after 20 m... | Bioorg Med Chem 26: 3696-3706 (2018) Article DOI: 10.1016/j.bmc.2018.05.050 BindingDB Entry DOI: 10.7270/Q2V69N32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50276043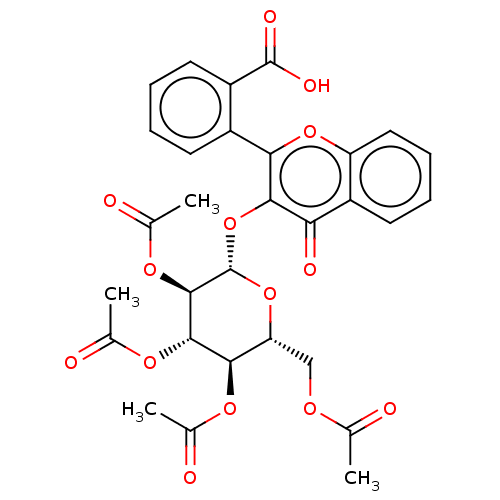 (CHEMBL4128409) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Gujrat Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate pretreated for 10 mins followed by substrate addition measured after 20 m... | Bioorg Med Chem 26: 3696-3706 (2018) Article DOI: 10.1016/j.bmc.2018.05.050 BindingDB Entry DOI: 10.7270/Q2V69N32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50276067 (CHEMBL4129776) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Gujrat Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate pretreated for 10 mins followed by substrate addition measured after 20 m... | Bioorg Med Chem 26: 3696-3706 (2018) Article DOI: 10.1016/j.bmc.2018.05.050 BindingDB Entry DOI: 10.7270/Q2V69N32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50276066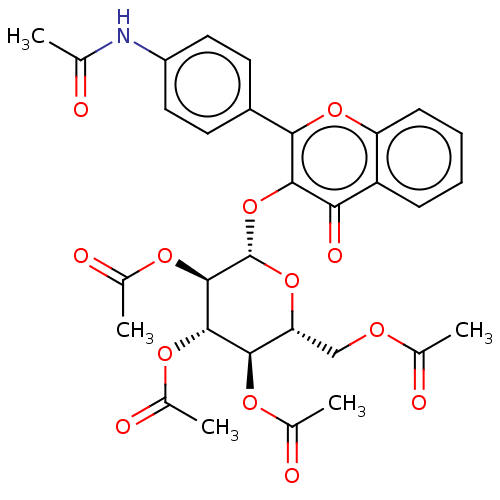 (CHEMBL4128492) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.87E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Gujrat Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate pretreated for 10 mins followed by substrate addition measured after 20 m... | Bioorg Med Chem 26: 3696-3706 (2018) Article DOI: 10.1016/j.bmc.2018.05.050 BindingDB Entry DOI: 10.7270/Q2V69N32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50276066 (CHEMBL4128492) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Gujrat Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine iodide as substrate pretreated for 10 mins followed by substrate addition measured after 20 m... | Bioorg Med Chem 26: 3696-3706 (2018) Article DOI: 10.1016/j.bmc.2018.05.050 BindingDB Entry DOI: 10.7270/Q2V69N32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50276060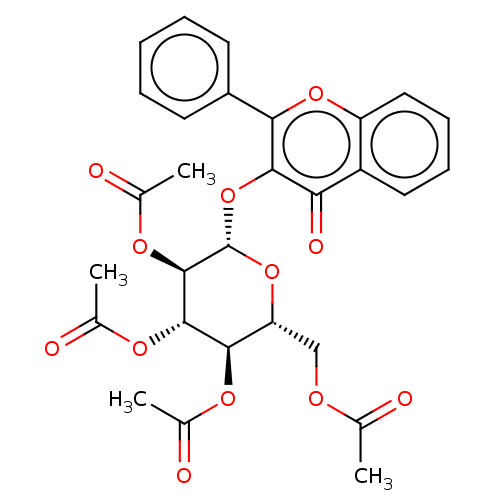 (CHEMBL4128639) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Gujrat Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine iodide as substrate pretreated for 10 mins followed by substrate addition measured after 20 m... | Bioorg Med Chem 26: 3696-3706 (2018) Article DOI: 10.1016/j.bmc.2018.05.050 BindingDB Entry DOI: 10.7270/Q2V69N32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50276043 (CHEMBL4128409) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Gujrat Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine iodide as substrate pretreated for 10 mins followed by substrate addition measured after 20 m... | Bioorg Med Chem 26: 3696-3706 (2018) Article DOI: 10.1016/j.bmc.2018.05.050 BindingDB Entry DOI: 10.7270/Q2V69N32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50276062 (CHEMBL4129855) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Gujrat Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine iodide as substrate pretreated for 10 mins followed by substrate addition measured after 20 m... | Bioorg Med Chem 26: 3696-3706 (2018) Article DOI: 10.1016/j.bmc.2018.05.050 BindingDB Entry DOI: 10.7270/Q2V69N32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Homo sapiens (Human)) | BDBM50500809 (CHEMBL3759529) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of HDAC in human HeLa cell nuclear extract using BML-KI104 Fluor de Lys as substrate by fluorescence-based assay | J Nat Prod 78: 2867-79 (2015) Article DOI: 10.1021/acs.jnatprod.5b00700 BindingDB Entry DOI: 10.7270/Q2PG1VR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50276060 (CHEMBL4128639) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.03E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Gujrat Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate pretreated for 10 mins followed by substrate addition measured after 20 m... | Bioorg Med Chem 26: 3696-3706 (2018) Article DOI: 10.1016/j.bmc.2018.05.050 BindingDB Entry DOI: 10.7270/Q2V69N32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Homo sapiens (Human)) | BDBM50500811 (CHEMBL3759749) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.22E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of HDAC in human HeLa cell nuclear extract using BML-KI104 Fluor de Lys as substrate by fluorescence-based assay | J Nat Prod 78: 2867-79 (2015) Article DOI: 10.1021/acs.jnatprod.5b00700 BindingDB Entry DOI: 10.7270/Q2PG1VR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Homo sapiens (Human)) | BDBM50140172 (CHEBI:3962 | CHEMBL140 | Curcumin | US9409845, Tab...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.87E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of HDAC in human HeLa cell nuclear extract using BML-KI104 Fluor de Lys as substrate by fluorescence-based assay | J Nat Prod 78: 2867-79 (2015) Article DOI: 10.1021/acs.jnatprod.5b00700 BindingDB Entry DOI: 10.7270/Q2PG1VR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Homo sapiens (Human)) | BDBM50500812 (CHEMBL3758253) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.88E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of HDAC in human HeLa cell nuclear extract using BML-KI104 Fluor de Lys as substrate by fluorescence-based assay | J Nat Prod 78: 2867-79 (2015) Article DOI: 10.1021/acs.jnatprod.5b00700 BindingDB Entry DOI: 10.7270/Q2PG1VR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Homo sapiens (Human)) | BDBM50500816 (CHEMBL3758656) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.17E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of HDAC in human HeLa cell nuclear extract using BML-KI104 Fluor de Lys as substrate by fluorescence-based assay | J Nat Prod 78: 2867-79 (2015) Article DOI: 10.1021/acs.jnatprod.5b00700 BindingDB Entry DOI: 10.7270/Q2PG1VR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Homo sapiens (Human)) | BDBM50500814 (CHEMBL1087807) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.47E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of HDAC in human HeLa cell nuclear extract using BML-KI104 Fluor de Lys as substrate by fluorescence-based assay | J Nat Prod 78: 2867-79 (2015) Article DOI: 10.1021/acs.jnatprod.5b00700 BindingDB Entry DOI: 10.7270/Q2PG1VR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Homo sapiens (Human)) | BDBM50500810 (CHEMBL3758791) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.95E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of HDAC in human HeLa cell nuclear extract using BML-KI104 Fluor de Lys as substrate by fluorescence-based assay | J Nat Prod 78: 2867-79 (2015) Article DOI: 10.1021/acs.jnatprod.5b00700 BindingDB Entry DOI: 10.7270/Q2PG1VR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Homo sapiens (Human)) | BDBM50500815 (CHEMBL3759699) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.86E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of HDAC in human HeLa cell nuclear extract using BML-KI104 Fluor de Lys as substrate by fluorescence-based assay | J Nat Prod 78: 2867-79 (2015) Article DOI: 10.1021/acs.jnatprod.5b00700 BindingDB Entry DOI: 10.7270/Q2PG1VR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Homo sapiens (Human)) | BDBM50500813 (CHEMBL1087690) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.62E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of HDAC in human HeLa cell nuclear extract using BML-KI104 Fluor de Lys as substrate by fluorescence-based assay | J Nat Prod 78: 2867-79 (2015) Article DOI: 10.1021/acs.jnatprod.5b00700 BindingDB Entry DOI: 10.7270/Q2PG1VR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||