 Found 278 hits with Last Name = 'kois' and Initial = 'a'
Found 278 hits with Last Name = 'kois' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50288051

(5-Dimethylamino-naphthalene-1-sulfonic acid (4-bro...)Show SMILES CN(C)c1cccc2c(cccc12)S(=O)(=O)Nc1onc(C)c1Br Show InChI InChI=1S/C16H16BrN3O3S/c1-10-15(17)16(23-18-10)19-24(21,22)14-9-5-6-11-12(14)7-4-8-13(11)20(2)3/h4-9,19H,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against human ETA receptor in TE 671(ATCC# HTB 139) cell membrane preparation |
Bioorg Med Chem Lett 6: 2393-2398 (1996)
Article DOI: 10.1016/0960-894X(96)00441-6
BindingDB Entry DOI: 10.7270/Q22V2G3R |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50288038
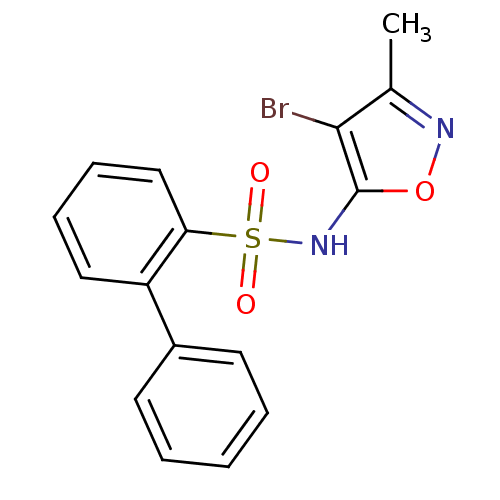
(Biphenyl-2-sulfonic acid (4-bromo-3-methyl-isoxazo...)Show InChI InChI=1S/C16H13BrN2O3S/c1-11-15(17)16(22-18-11)19-23(20,21)14-10-6-5-9-13(14)12-7-3-2-4-8-12/h2-10,19H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against human Endothelin A receptor in TE 671(ATCC# HTB 139) cell membrane preparation |
Bioorg Med Chem Lett 6: 2393-2398 (1996)
Article DOI: 10.1016/0960-894X(96)00441-6
BindingDB Entry DOI: 10.7270/Q22V2G3R |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50288013
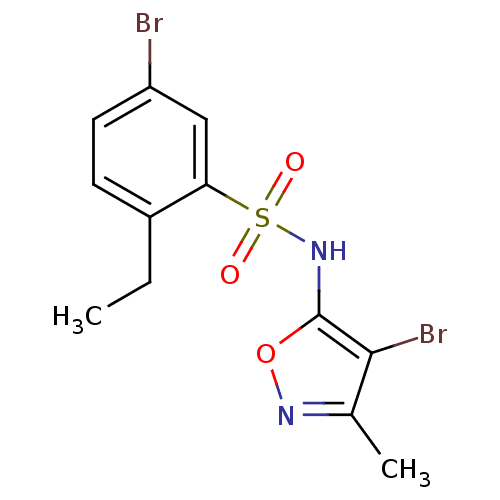
(5-Bromo-N-(4-bromo-3-methyl-isoxazol-5-yl)-2-ethyl...)Show InChI InChI=1S/C12H12Br2N2O3S/c1-3-8-4-5-9(13)6-10(8)20(17,18)16-12-11(14)7(2)15-19-12/h4-6,16H,3H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against human Endothelin A receptor in TE 671(ATCC# HTB 139) cell membrane preparation |
Bioorg Med Chem Lett 6: 2393-2398 (1996)
Article DOI: 10.1016/0960-894X(96)00441-6
BindingDB Entry DOI: 10.7270/Q22V2G3R |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50288040
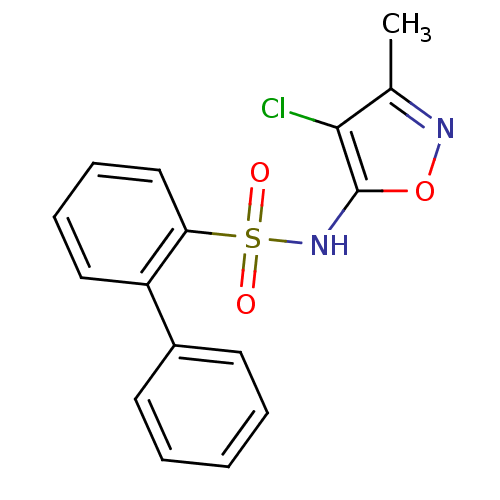
(Biphenyl-2-sulfonic acid (4-chloro-3-methyl-isoxaz...)Show InChI InChI=1S/C16H13ClN2O3S/c1-11-15(17)16(22-18-11)19-23(20,21)14-10-6-5-9-13(14)12-7-3-2-4-8-12/h2-10,19H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against human Endothelin A receptor in TE 671(ATCC# HTB 139) cell membrane preparation |
Bioorg Med Chem Lett 6: 2393-2398 (1996)
Article DOI: 10.1016/0960-894X(96)00441-6
BindingDB Entry DOI: 10.7270/Q22V2G3R |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50034435
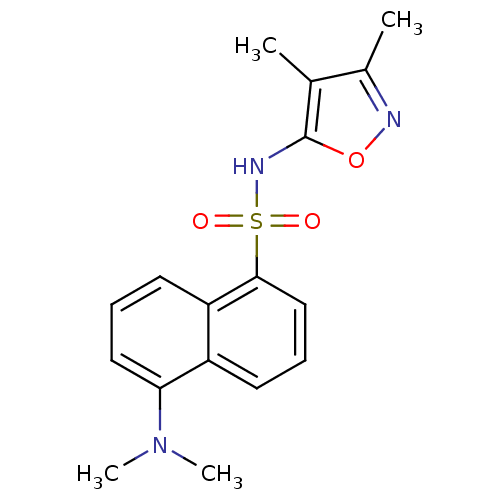
(5-Dimethylamino-naphthalene-1-sulfonic acid (3,4-d...)Show InChI InChI=1S/C17H19N3O3S/c1-11-12(2)18-23-17(11)19-24(21,22)16-10-6-7-13-14(16)8-5-9-15(13)20(3)4/h5-10,19H,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against human ETA receptor in TE 671(ATCC# HTB 139) cell membrane preparation |
Bioorg Med Chem Lett 6: 2393-2398 (1996)
Article DOI: 10.1016/0960-894X(96)00441-6
BindingDB Entry DOI: 10.7270/Q22V2G3R |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50288007
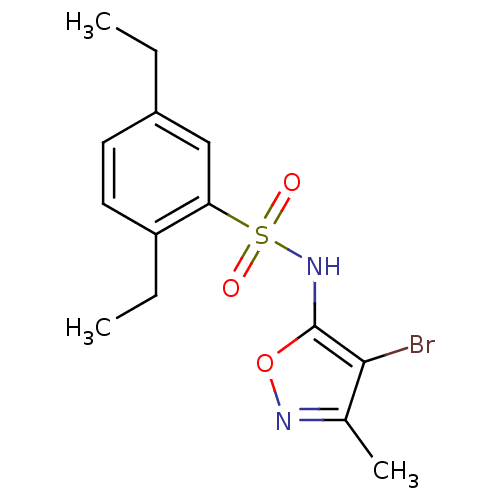
(CHEMBL311138 | N-(4-Bromo-3-methyl-isoxazol-5-yl)-...)Show InChI InChI=1S/C14H17BrN2O3S/c1-4-10-6-7-11(5-2)12(8-10)21(18,19)17-14-13(15)9(3)16-20-14/h6-8,17H,4-5H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against human Endothelin A receptor in TE 671(ATCC# HTB 139) cell membrane preparation |
Bioorg Med Chem Lett 6: 2393-2398 (1996)
Article DOI: 10.1016/0960-894X(96)00441-6
BindingDB Entry DOI: 10.7270/Q22V2G3R |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50288045
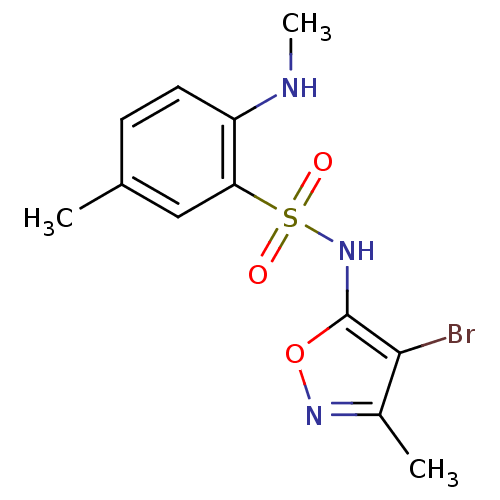
(CHEMBL78563 | N-(4-Bromo-3-methyl-isoxazol-5-yl)-5...)Show InChI InChI=1S/C12H14BrN3O3S/c1-7-4-5-9(14-3)10(6-7)20(17,18)16-12-11(13)8(2)15-19-12/h4-6,14,16H,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against human Endothelin A receptor in TE 671(ATCC# HTB 139) cell membrane preparation |
Bioorg Med Chem Lett 6: 2393-2398 (1996)
Article DOI: 10.1016/0960-894X(96)00441-6
BindingDB Entry DOI: 10.7270/Q22V2G3R |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50068700
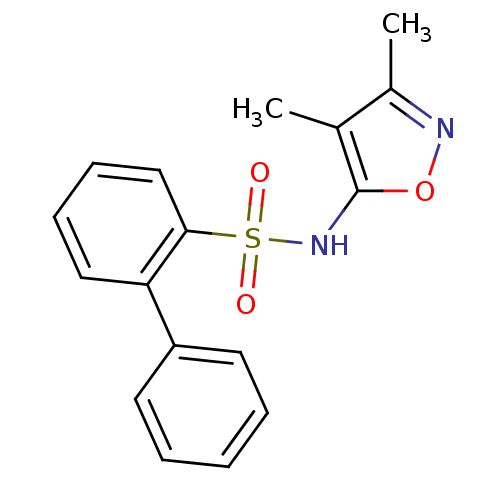
(Biphenyl-2-sulfonic acid (3,4-dimethyl-isoxazol-5-...)Show InChI InChI=1S/C17H16N2O3S/c1-12-13(2)18-22-17(12)19-23(20,21)16-11-7-6-10-15(16)14-8-4-3-5-9-14/h3-11,19H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against human ETA receptor in TE 671(ATCC# HTB 139) cell membrane preparation |
Bioorg Med Chem Lett 6: 2393-2398 (1996)
Article DOI: 10.1016/0960-894X(96)00441-6
BindingDB Entry DOI: 10.7270/Q22V2G3R |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50363465
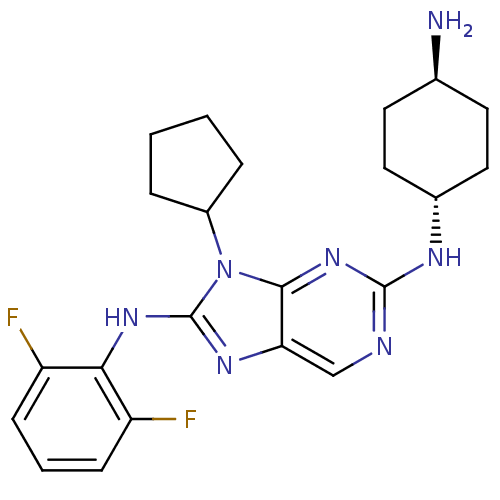
(CHEMBL1946646)Show SMILES N[C@H]1CC[C@@H](CC1)Nc1ncc2nc(Nc3c(F)cccc3F)n(C3CCCC3)c2n1 |r,wU:4.7,wD:1.0,(-8.05,-2.1,;-7.28,-.77,;-5.74,-.77,;-4.96,.56,;-5.74,1.89,;-7.27,1.89,;-8.04,.57,;-4.97,3.22,;-3.43,3.22,;-2.66,4.56,;-1.12,4.55,;-.36,3.22,;1.14,2.91,;1.29,1.38,;2.63,.61,;3.96,1.38,;3.96,2.92,;2.63,3.69,;5.29,3.7,;6.63,2.93,;6.63,1.38,;5.3,.61,;5.3,-.93,;-.11,.75,;-.11,-.78,;-1.36,-1.68,;-.89,-3.15,;.65,-3.15,;1.13,-1.69,;-1.14,1.9,;-2.66,1.9,)| Show InChI InChI=1S/C22H27F2N7/c23-16-6-3-7-17(24)19(16)29-22-28-18-12-26-21(27-14-10-8-13(25)9-11-14)30-20(18)31(22)15-4-1-2-5-15/h3,6-7,12-15H,1-2,4-5,8-11,25H2,(H,28,29)(H,26,27,30)/t13-,14- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of full length Hexa-His-tagged JNK2 using GST-tagged cJun and [gamma33P]ATP as substrate after 60 mins by scintillation counting |
Bioorg Med Chem Lett 22: 1427-32 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.028
BindingDB Entry DOI: 10.7270/Q2513ZP6 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50363455
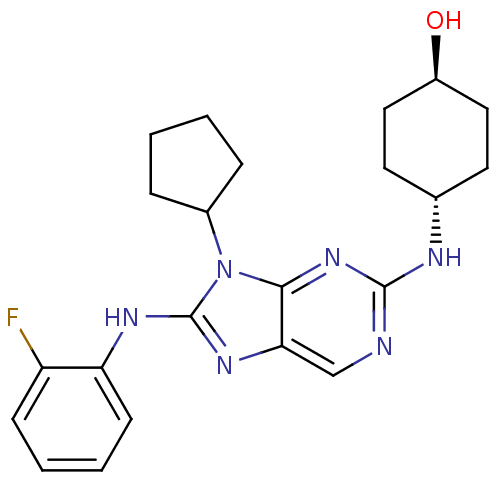
(CHEMBL1946333)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1ncc2nc(Nc3ccccc3F)n(C3CCCC3)c2n1 |r,wU:1.0,wD:4.7,(-11.53,-21.51,;-10.75,-20.18,;-11.51,-18.84,;-10.74,-17.51,;-9.2,-17.52,;-8.43,-18.84,;-9.2,-20.18,;-8.44,-16.18,;-6.9,-16.18,;-6.13,-14.85,;-4.59,-14.85,;-3.83,-16.18,;-2.33,-16.49,;-2.17,-18.02,;-.84,-18.79,;.49,-18.03,;1.83,-18.8,;3.16,-18.02,;3.16,-16.47,;1.82,-15.71,;.5,-16.48,;-.84,-15.71,;-3.58,-18.65,;-3.58,-20.19,;-4.83,-21.09,;-4.36,-22.55,;-2.82,-22.56,;-2.34,-21.1,;-4.6,-17.51,;-6.13,-17.51,)| Show InChI InChI=1S/C22H27FN6O/c23-17-7-3-4-8-18(17)26-22-27-19-13-24-21(25-14-9-11-16(30)12-10-14)28-20(19)29(22)15-5-1-2-6-15/h3-4,7-8,13-16,30H,1-2,5-6,9-12H2,(H,26,27)(H,24,25,28)/t14-,16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of full length Hexa-His-tagged JNK2 using GST-tagged cJun and [gamma33P]ATP as substrate after 60 mins by scintillation counting |
Bioorg Med Chem Lett 22: 1427-32 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.028
BindingDB Entry DOI: 10.7270/Q2513ZP6 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50363466
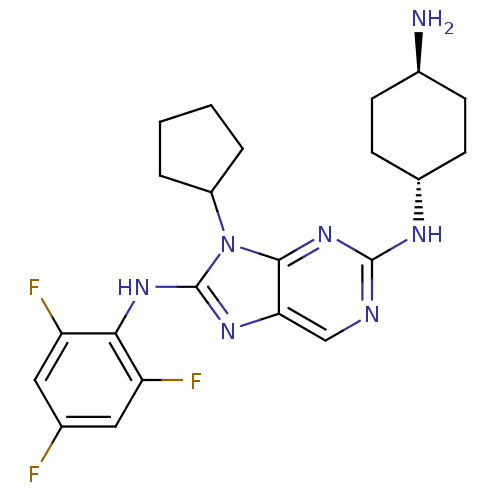
(CHEMBL1946647)Show SMILES N[C@H]1CC[C@@H](CC1)Nc1ncc2nc(Nc3c(F)cc(F)cc3F)n(C3CCCC3)c2n1 |r,wU:4.7,wD:1.0,(9.91,-2.33,;10.68,-1,;12.22,-1.01,;13,.33,;12.22,1.65,;10.69,1.66,;9.92,.33,;12.99,2.99,;14.53,2.99,;15.3,4.32,;16.83,4.32,;17.6,2.99,;19.1,2.68,;19.25,1.15,;20.59,.38,;21.92,1.15,;21.92,2.69,;20.59,3.46,;23.25,3.46,;24.59,2.7,;25.92,3.47,;24.59,1.15,;23.26,.38,;23.25,-1.16,;17.85,.52,;17.85,-1.02,;16.6,-1.92,;17.07,-3.38,;18.61,-3.39,;19.09,-1.93,;16.82,1.67,;15.3,1.67,)| Show InChI InChI=1S/C22H26F3N7/c23-12-9-16(24)19(17(25)10-12)30-22-29-18-11-27-21(28-14-7-5-13(26)6-8-14)31-20(18)32(22)15-3-1-2-4-15/h9-11,13-15H,1-8,26H2,(H,29,30)(H,27,28,31)/t13-,14- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of full length Hexa-His-tagged JNK2 using GST-tagged cJun and [gamma33P]ATP as substrate after 60 mins by scintillation counting |
Bioorg Med Chem Lett 22: 1427-32 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.028
BindingDB Entry DOI: 10.7270/Q2513ZP6 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50363455

(CHEMBL1946333)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1ncc2nc(Nc3ccccc3F)n(C3CCCC3)c2n1 |r,wU:1.0,wD:4.7,(-11.53,-21.51,;-10.75,-20.18,;-11.51,-18.84,;-10.74,-17.51,;-9.2,-17.52,;-8.43,-18.84,;-9.2,-20.18,;-8.44,-16.18,;-6.9,-16.18,;-6.13,-14.85,;-4.59,-14.85,;-3.83,-16.18,;-2.33,-16.49,;-2.17,-18.02,;-.84,-18.79,;.49,-18.03,;1.83,-18.8,;3.16,-18.02,;3.16,-16.47,;1.82,-15.71,;.5,-16.48,;-.84,-15.71,;-3.58,-18.65,;-3.58,-20.19,;-4.83,-21.09,;-4.36,-22.55,;-2.82,-22.56,;-2.34,-21.1,;-4.6,-17.51,;-6.13,-17.51,)| Show InChI InChI=1S/C22H27FN6O/c23-17-7-3-4-8-18(17)26-22-27-19-13-24-21(25-14-9-11-16(30)12-10-14)28-20(19)29(22)15-5-1-2-6-15/h3-4,7-8,13-16,30H,1-2,5-6,9-12H2,(H,26,27)(H,24,25,28)/t14-,16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of full length Hexa-His-tagged JNK3 using GST-tagged cJun and [gamma33P]ATP as substrate after 60 mins by scintillation counting |
Bioorg Med Chem Lett 22: 1427-32 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.028
BindingDB Entry DOI: 10.7270/Q2513ZP6 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50363465

(CHEMBL1946646)Show SMILES N[C@H]1CC[C@@H](CC1)Nc1ncc2nc(Nc3c(F)cccc3F)n(C3CCCC3)c2n1 |r,wU:4.7,wD:1.0,(-8.05,-2.1,;-7.28,-.77,;-5.74,-.77,;-4.96,.56,;-5.74,1.89,;-7.27,1.89,;-8.04,.57,;-4.97,3.22,;-3.43,3.22,;-2.66,4.56,;-1.12,4.55,;-.36,3.22,;1.14,2.91,;1.29,1.38,;2.63,.61,;3.96,1.38,;3.96,2.92,;2.63,3.69,;5.29,3.7,;6.63,2.93,;6.63,1.38,;5.3,.61,;5.3,-.93,;-.11,.75,;-.11,-.78,;-1.36,-1.68,;-.89,-3.15,;.65,-3.15,;1.13,-1.69,;-1.14,1.9,;-2.66,1.9,)| Show InChI InChI=1S/C22H27F2N7/c23-16-6-3-7-17(24)19(16)29-22-28-18-12-26-21(27-14-10-8-13(25)9-11-14)30-20(18)31(22)15-4-1-2-5-15/h3,6-7,12-15H,1-2,4-5,8-11,25H2,(H,28,29)(H,26,27,30)/t13-,14- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of full length Hexa-His-tagged JNK3 using GST-tagged cJun and [gamma33P]ATP as substrate after 60 mins by scintillation counting |
Bioorg Med Chem Lett 22: 1427-32 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.028
BindingDB Entry DOI: 10.7270/Q2513ZP6 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50363462
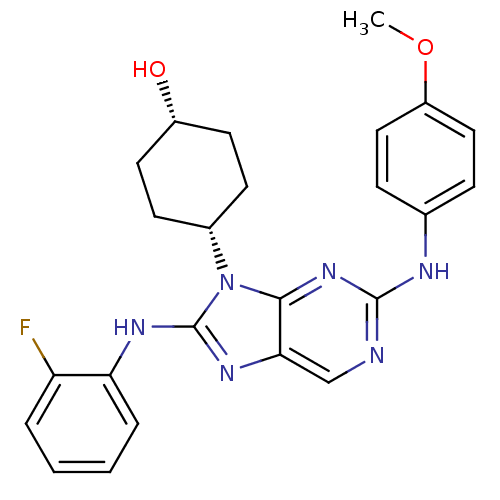
(CHEMBL1946340)Show SMILES COc1ccc(Nc2ncc3nc(Nc4ccccc4F)n([C@@H]4CC[C@H](O)CC4)c3n2)cc1 |r,wU:22.22,25.26,(4.81,-38.68,;6.35,-38.68,;7.12,-37.34,;6.35,-36.01,;7.12,-34.67,;8.66,-34.68,;9.43,-33.35,;10.97,-33.34,;11.74,-32.01,;13.27,-32.01,;14.04,-33.34,;15.54,-33.66,;15.7,-35.19,;17.03,-35.96,;18.37,-35.19,;19.7,-35.96,;21.04,-35.19,;21.04,-33.64,;19.7,-32.87,;18.37,-33.64,;17.03,-32.87,;14.29,-35.82,;14.29,-37.35,;12.95,-38.11,;12.94,-39.64,;14.27,-40.42,;14.26,-41.96,;15.61,-39.66,;15.62,-38.11,;13.26,-34.67,;11.74,-34.67,;9.43,-36,;8.67,-37.34,)| Show InChI InChI=1S/C24H25FN6O2/c1-33-18-12-6-15(7-13-18)27-23-26-14-21-22(30-23)31(16-8-10-17(32)11-9-16)24(29-21)28-20-5-3-2-4-19(20)25/h2-7,12-14,16-17,32H,8-11H2,1H3,(H,28,29)(H,26,27,30)/t16-,17+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of full length Hexa-His-tagged JNK2 using GST-tagged cJun and [gamma33P]ATP as substrate after 60 mins by scintillation counting |
Bioorg Med Chem Lett 22: 1427-32 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.028
BindingDB Entry DOI: 10.7270/Q2513ZP6 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50288038

(Biphenyl-2-sulfonic acid (4-bromo-3-methyl-isoxazo...)Show InChI InChI=1S/C16H13BrN2O3S/c1-11-15(17)16(22-18-11)19-23(20,21)14-10-6-5-9-13(14)12-7-3-2-4-8-12/h2-10,19H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for binding affinity against Endothelin A receptor |
Bioorg Med Chem Lett 6: 2651-2656 (1996)
Article DOI: 10.1016/S0960-894X(96)00496-9
BindingDB Entry DOI: 10.7270/Q24M94HV |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50363466

(CHEMBL1946647)Show SMILES N[C@H]1CC[C@@H](CC1)Nc1ncc2nc(Nc3c(F)cc(F)cc3F)n(C3CCCC3)c2n1 |r,wU:4.7,wD:1.0,(9.91,-2.33,;10.68,-1,;12.22,-1.01,;13,.33,;12.22,1.65,;10.69,1.66,;9.92,.33,;12.99,2.99,;14.53,2.99,;15.3,4.32,;16.83,4.32,;17.6,2.99,;19.1,2.68,;19.25,1.15,;20.59,.38,;21.92,1.15,;21.92,2.69,;20.59,3.46,;23.25,3.46,;24.59,2.7,;25.92,3.47,;24.59,1.15,;23.26,.38,;23.25,-1.16,;17.85,.52,;17.85,-1.02,;16.6,-1.92,;17.07,-3.38,;18.61,-3.39,;19.09,-1.93,;16.82,1.67,;15.3,1.67,)| Show InChI InChI=1S/C22H26F3N7/c23-12-9-16(24)19(17(25)10-12)30-22-29-18-11-27-21(28-14-7-5-13(26)6-8-14)31-20(18)32(22)15-3-1-2-4-15/h9-11,13-15H,1-8,26H2,(H,29,30)(H,27,28,31)/t13-,14- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of full length Hexa-His-tagged JNK3 using GST-tagged cJun and [gamma33P]ATP as substrate after 60 mins by scintillation counting |
Bioorg Med Chem Lett 22: 1427-32 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.028
BindingDB Entry DOI: 10.7270/Q2513ZP6 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50287992

(5-Bromo-N-(3,4-dimethyl-isoxazol-5-yl)-2-propyl-be...)Show InChI InChI=1S/C14H17BrN2O3S/c1-4-5-11-6-7-12(15)8-13(11)21(18,19)17-14-9(2)10(3)16-20-14/h6-8,17H,4-5H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against human Endothelin A receptor in TE 671(ATCC# HTB 139) cell membrane preparation |
Bioorg Med Chem Lett 6: 2393-2398 (1996)
Article DOI: 10.1016/0960-894X(96)00441-6
BindingDB Entry DOI: 10.7270/Q22V2G3R |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50363462

(CHEMBL1946340)Show SMILES COc1ccc(Nc2ncc3nc(Nc4ccccc4F)n([C@@H]4CC[C@H](O)CC4)c3n2)cc1 |r,wU:22.22,25.26,(4.81,-38.68,;6.35,-38.68,;7.12,-37.34,;6.35,-36.01,;7.12,-34.67,;8.66,-34.68,;9.43,-33.35,;10.97,-33.34,;11.74,-32.01,;13.27,-32.01,;14.04,-33.34,;15.54,-33.66,;15.7,-35.19,;17.03,-35.96,;18.37,-35.19,;19.7,-35.96,;21.04,-35.19,;21.04,-33.64,;19.7,-32.87,;18.37,-33.64,;17.03,-32.87,;14.29,-35.82,;14.29,-37.35,;12.95,-38.11,;12.94,-39.64,;14.27,-40.42,;14.26,-41.96,;15.61,-39.66,;15.62,-38.11,;13.26,-34.67,;11.74,-34.67,;9.43,-36,;8.67,-37.34,)| Show InChI InChI=1S/C24H25FN6O2/c1-33-18-12-6-15(7-13-18)27-23-26-14-21-22(30-23)31(16-8-10-17(32)11-9-16)24(29-21)28-20-5-3-2-4-19(20)25/h2-7,12-14,16-17,32H,8-11H2,1H3,(H,28,29)(H,26,27,30)/t16-,17+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of full length Hexa-His-tagged JNK3 using GST-tagged cJun and [gamma33P]ATP as substrate after 60 mins by scintillation counting |
Bioorg Med Chem Lett 22: 1427-32 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.028
BindingDB Entry DOI: 10.7270/Q2513ZP6 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50363464
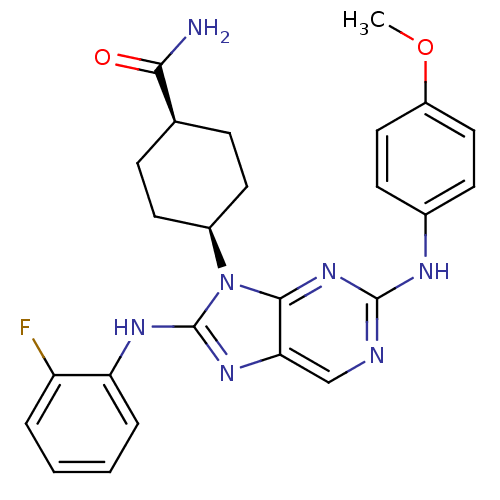
(CHEMBL1946495)Show SMILES COc1ccc(Nc2ncc3nc(Nc4ccccc4F)n([C@@H]4CC[C@@H](CC4)C(N)=O)c3n2)cc1 |r,wU:22.22,25.29,(14.63,-45.79,;16.17,-45.79,;16.94,-44.45,;16.17,-43.11,;16.94,-41.78,;18.48,-41.79,;19.25,-40.45,;20.79,-40.45,;21.56,-39.12,;23.09,-39.12,;23.86,-40.45,;25.36,-40.76,;25.52,-42.29,;26.85,-43.06,;28.19,-42.29,;29.52,-43.07,;30.86,-42.29,;30.85,-40.74,;29.52,-39.98,;28.19,-40.75,;26.85,-39.98,;24.11,-42.92,;24.11,-44.46,;22.77,-45.21,;22.77,-46.74,;24.09,-47.52,;25.43,-46.76,;25.44,-45.22,;24.08,-49.06,;25.41,-49.84,;22.74,-49.83,;23.08,-41.77,;21.56,-41.77,;19.25,-43.11,;18.49,-44.44,)| Show InChI InChI=1S/C25H26FN7O2/c1-35-18-12-8-16(9-13-18)29-24-28-14-21-23(32-24)33(17-10-6-15(7-11-17)22(27)34)25(31-21)30-20-5-3-2-4-19(20)26/h2-5,8-9,12-15,17H,6-7,10-11H2,1H3,(H2,27,34)(H,30,31)(H,28,29,32)/t15-,17+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of full length Hexa-His-tagged JNK2 using GST-tagged cJun and [gamma33P]ATP as substrate after 60 mins by scintillation counting |
Bioorg Med Chem Lett 22: 1427-32 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.028
BindingDB Entry DOI: 10.7270/Q2513ZP6 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50363464

(CHEMBL1946495)Show SMILES COc1ccc(Nc2ncc3nc(Nc4ccccc4F)n([C@@H]4CC[C@@H](CC4)C(N)=O)c3n2)cc1 |r,wU:22.22,25.29,(14.63,-45.79,;16.17,-45.79,;16.94,-44.45,;16.17,-43.11,;16.94,-41.78,;18.48,-41.79,;19.25,-40.45,;20.79,-40.45,;21.56,-39.12,;23.09,-39.12,;23.86,-40.45,;25.36,-40.76,;25.52,-42.29,;26.85,-43.06,;28.19,-42.29,;29.52,-43.07,;30.86,-42.29,;30.85,-40.74,;29.52,-39.98,;28.19,-40.75,;26.85,-39.98,;24.11,-42.92,;24.11,-44.46,;22.77,-45.21,;22.77,-46.74,;24.09,-47.52,;25.43,-46.76,;25.44,-45.22,;24.08,-49.06,;25.41,-49.84,;22.74,-49.83,;23.08,-41.77,;21.56,-41.77,;19.25,-43.11,;18.49,-44.44,)| Show InChI InChI=1S/C25H26FN7O2/c1-35-18-12-8-16(9-13-18)29-24-28-14-21-23(32-24)33(17-10-6-15(7-11-17)22(27)34)25(31-21)30-20-5-3-2-4-19(20)26/h2-5,8-9,12-15,17H,6-7,10-11H2,1H3,(H2,27,34)(H,30,31)(H,28,29,32)/t15-,17+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of full length Hexa-His-tagged JNK3 using GST-tagged cJun and [gamma33P]ATP as substrate after 60 mins by scintillation counting |
Bioorg Med Chem Lett 22: 1427-32 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.028
BindingDB Entry DOI: 10.7270/Q2513ZP6 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50363467

(CHEMBL1946837)Show SMILES N[C@H]1CC[C@@H](CC1)Nc1ncc2nc(Nc3ccc(F)cc3F)n(C3CCCC3)c2n1 |r,wU:4.7,wD:1.0,(-5.55,-13.29,;-4.78,-11.95,;-3.24,-11.96,;-2.46,-10.62,;-3.24,-9.3,;-4.78,-9.29,;-5.55,-10.62,;-2.47,-7.96,;-.93,-7.96,;-.16,-6.63,;1.37,-6.63,;2.13,-7.96,;3.64,-8.27,;3.79,-9.8,;5.12,-10.57,;6.46,-9.8,;7.79,-10.58,;9.13,-9.8,;9.12,-8.25,;10.46,-7.48,;7.79,-7.49,;6.46,-8.26,;5.13,-7.49,;2.39,-10.43,;2.39,-11.97,;1.14,-12.87,;1.61,-14.33,;3.15,-14.34,;3.63,-12.88,;1.36,-9.29,;-.16,-9.28,)| Show InChI InChI=1S/C22H27F2N7/c23-13-5-10-18(17(24)11-13)28-22-29-19-12-26-21(27-15-8-6-14(25)7-9-15)30-20(19)31(22)16-3-1-2-4-16/h5,10-12,14-16H,1-4,6-9,25H2,(H,28,29)(H,26,27,30)/t14-,15- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of full length Hexa-His-tagged JNK3 using GST-tagged cJun and [gamma33P]ATP as substrate after 60 mins by scintillation counting |
Bioorg Med Chem Lett 22: 1427-32 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.028
BindingDB Entry DOI: 10.7270/Q2513ZP6 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50363467

(CHEMBL1946837)Show SMILES N[C@H]1CC[C@@H](CC1)Nc1ncc2nc(Nc3ccc(F)cc3F)n(C3CCCC3)c2n1 |r,wU:4.7,wD:1.0,(-5.55,-13.29,;-4.78,-11.95,;-3.24,-11.96,;-2.46,-10.62,;-3.24,-9.3,;-4.78,-9.29,;-5.55,-10.62,;-2.47,-7.96,;-.93,-7.96,;-.16,-6.63,;1.37,-6.63,;2.13,-7.96,;3.64,-8.27,;3.79,-9.8,;5.12,-10.57,;6.46,-9.8,;7.79,-10.58,;9.13,-9.8,;9.12,-8.25,;10.46,-7.48,;7.79,-7.49,;6.46,-8.26,;5.13,-7.49,;2.39,-10.43,;2.39,-11.97,;1.14,-12.87,;1.61,-14.33,;3.15,-14.34,;3.63,-12.88,;1.36,-9.29,;-.16,-9.28,)| Show InChI InChI=1S/C22H27F2N7/c23-13-5-10-18(17(24)11-13)28-22-29-19-12-26-21(27-15-8-6-14(25)7-9-15)30-20(19)31(22)16-3-1-2-4-16/h5,10-12,14-16H,1-4,6-9,25H2,(H,28,29)(H,26,27,30)/t14-,15- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of full length Hexa-His-tagged JNK2 using GST-tagged cJun and [gamma33P]ATP as substrate after 60 mins by scintillation counting |
Bioorg Med Chem Lett 22: 1427-32 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.028
BindingDB Entry DOI: 10.7270/Q2513ZP6 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50363456
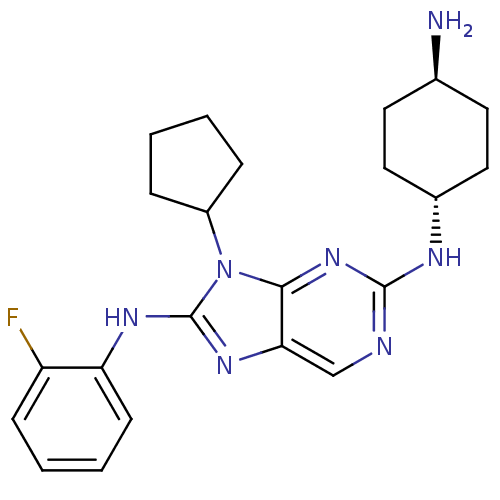
(CHEMBL1946334)Show SMILES N[C@H]1CC[C@@H](CC1)Nc1ncc2nc(Nc3ccccc3F)n(C3CCCC3)c2n1 |r,wU:1.0,wD:4.7,(6.38,-21.6,;7.16,-20.27,;6.39,-18.93,;7.16,-17.6,;8.7,-17.61,;9.48,-18.94,;8.7,-20.27,;9.47,-16.28,;11.01,-16.28,;11.78,-14.94,;13.31,-14.95,;14.07,-16.28,;15.58,-16.59,;15.73,-18.12,;17.06,-18.89,;18.4,-18.12,;19.73,-18.89,;21.07,-18.12,;21.06,-16.57,;19.73,-15.8,;18.4,-16.57,;17.07,-15.8,;14.33,-18.75,;14.33,-20.28,;13.08,-21.18,;13.55,-22.65,;15.09,-22.65,;15.57,-21.19,;13.3,-17.6,;11.77,-17.6,)| Show InChI InChI=1S/C22H28FN7/c23-17-7-3-4-8-18(17)27-22-28-19-13-25-21(26-15-11-9-14(24)10-12-15)29-20(19)30(22)16-5-1-2-6-16/h3-4,7-8,13-16H,1-2,5-6,9-12,24H2,(H,27,28)(H,25,26,29)/t14-,15- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of full length Hexa-His-tagged JNK2 using GST-tagged cJun and [gamma33P]ATP as substrate after 60 mins by scintillation counting |
Bioorg Med Chem Lett 22: 1427-32 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.028
BindingDB Entry DOI: 10.7270/Q2513ZP6 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50288006
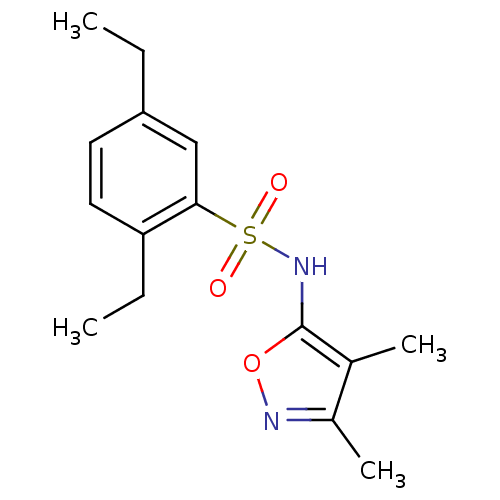
(CHEMBL79066 | N-(3,4-Dimethyl-isoxazol-5-yl)-2,5-d...)Show InChI InChI=1S/C15H20N2O3S/c1-5-12-7-8-13(6-2)14(9-12)21(18,19)17-15-10(3)11(4)16-20-15/h7-9,17H,5-6H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against human Endothelin A receptor in TE 671(ATCC# HTB 139) cell membrane preparation |
Bioorg Med Chem Lett 6: 2393-2398 (1996)
Article DOI: 10.1016/0960-894X(96)00441-6
BindingDB Entry DOI: 10.7270/Q22V2G3R |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50288032
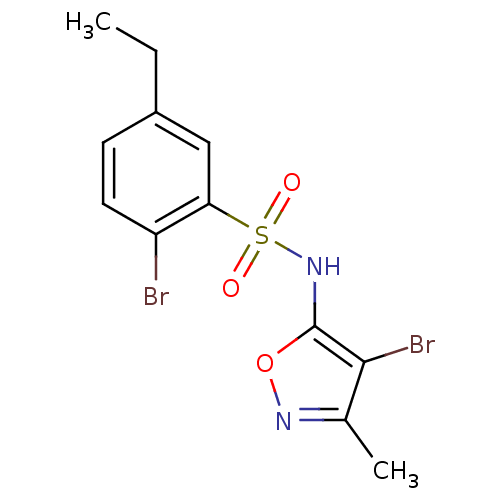
(2-Bromo-N-(4-bromo-3-methyl-isoxazol-5-yl)-5-ethyl...)Show InChI InChI=1S/C12H12Br2N2O3S/c1-3-8-4-5-9(13)10(6-8)20(17,18)16-12-11(14)7(2)15-19-12/h4-6,16H,3H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against human Endothelin A receptor in TE 671(ATCC# HTB 139) cell membrane preparation |
Bioorg Med Chem Lett 6: 2393-2398 (1996)
Article DOI: 10.1016/0960-894X(96)00441-6
BindingDB Entry DOI: 10.7270/Q22V2G3R |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50363456

(CHEMBL1946334)Show SMILES N[C@H]1CC[C@@H](CC1)Nc1ncc2nc(Nc3ccccc3F)n(C3CCCC3)c2n1 |r,wU:1.0,wD:4.7,(6.38,-21.6,;7.16,-20.27,;6.39,-18.93,;7.16,-17.6,;8.7,-17.61,;9.48,-18.94,;8.7,-20.27,;9.47,-16.28,;11.01,-16.28,;11.78,-14.94,;13.31,-14.95,;14.07,-16.28,;15.58,-16.59,;15.73,-18.12,;17.06,-18.89,;18.4,-18.12,;19.73,-18.89,;21.07,-18.12,;21.06,-16.57,;19.73,-15.8,;18.4,-16.57,;17.07,-15.8,;14.33,-18.75,;14.33,-20.28,;13.08,-21.18,;13.55,-22.65,;15.09,-22.65,;15.57,-21.19,;13.3,-17.6,;11.77,-17.6,)| Show InChI InChI=1S/C22H28FN7/c23-17-7-3-4-8-18(17)27-22-28-19-13-25-21(26-15-11-9-14(24)10-12-15)29-20(19)30(22)16-5-1-2-6-16/h3-4,7-8,13-16H,1-2,5-6,9-12,24H2,(H,27,28)(H,25,26,29)/t14-,15- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of full length Hexa-His-tagged JNK3 using GST-tagged cJun and [gamma33P]ATP as substrate after 60 mins by scintillation counting |
Bioorg Med Chem Lett 22: 1427-32 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.028
BindingDB Entry DOI: 10.7270/Q2513ZP6 |
More data for this
Ligand-Target Pair | |
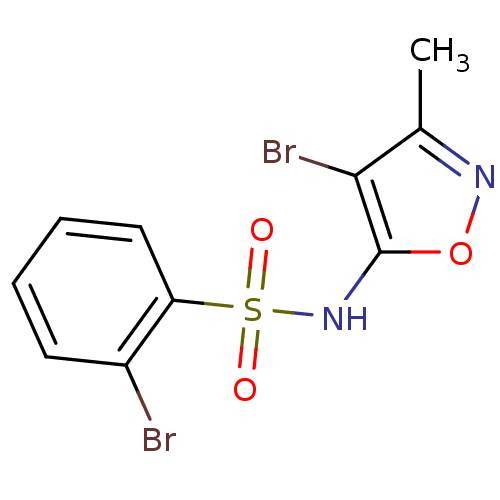
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50288017
(2-Bromo-N-(4-bromo-3-methyl-isoxazol-5-yl)-benzene...)Show InChI InChI=1S/C10H8Br2N2O3S/c1-6-9(12)10(17-13-6)14-18(15,16)8-5-3-2-4-7(8)11/h2-5,14H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against human Endothelin A receptor in TE 671(ATCC# HTB 139) cell membrane preparation |
Bioorg Med Chem Lett 6: 2393-2398 (1996)
Article DOI: 10.1016/0960-894X(96)00441-6
BindingDB Entry DOI: 10.7270/Q22V2G3R |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50288282
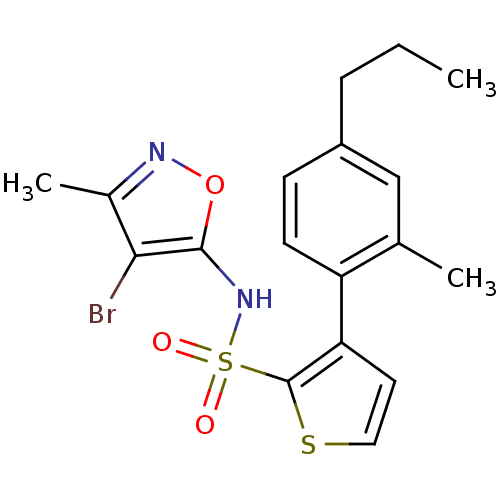
(3-(2-Methyl-4-propyl-phenyl)-thiophene-2-sulfonic ...)Show SMILES CCCc1ccc(-c2ccsc2S(=O)(=O)Nc2onc(C)c2Br)c(C)c1 Show InChI InChI=1S/C18H19BrN2O3S2/c1-4-5-13-6-7-14(11(2)10-13)15-8-9-25-18(15)26(22,23)21-17-16(19)12(3)20-24-17/h6-10,21H,4-5H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for binding affinity against Endothelin A receptor |
Bioorg Med Chem Lett 6: 2651-2656 (1996)
Article DOI: 10.1016/S0960-894X(96)00496-9
BindingDB Entry DOI: 10.7270/Q24M94HV |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50287994

(CHEMBL80484 | N-(4-Bromo-3-methyl-isoxazol-5-yl)-2...)Show InChI InChI=1S/C12H13BrN2O3S/c1-7-4-5-8(2)10(6-7)19(16,17)15-12-11(13)9(3)14-18-12/h4-6,15H,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against human Endothelin A receptor in TE 671(ATCC# HTB 139) cell membrane preparation |
Bioorg Med Chem Lett 6: 2393-2398 (1996)
Article DOI: 10.1016/0960-894X(96)00441-6
BindingDB Entry DOI: 10.7270/Q22V2G3R |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50363454
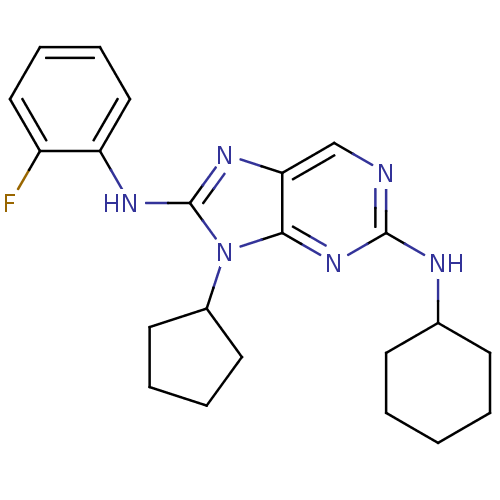
(CHEMBL1946332)Show InChI InChI=1S/C22H27FN6/c23-17-12-6-7-13-18(17)26-22-27-19-14-24-21(25-15-8-2-1-3-9-15)28-20(19)29(22)16-10-4-5-11-16/h6-7,12-16H,1-5,8-11H2,(H,26,27)(H,24,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of full length Hexa-His-tagged JNK2 using GST-tagged cJun and [gamma33P]ATP as substrate after 60 mins by scintillation counting |
Bioorg Med Chem Lett 22: 1427-32 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.028
BindingDB Entry DOI: 10.7270/Q2513ZP6 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50363450
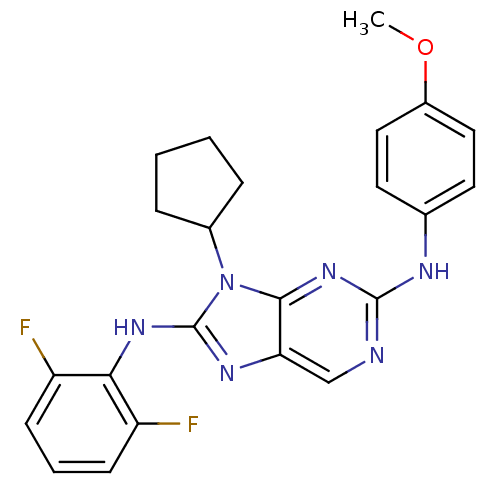
(CHEMBL1946328)Show SMILES COc1ccc(Nc2ncc3nc(Nc4c(F)cccc4F)n(C4CCCC4)c3n2)cc1 Show InChI InChI=1S/C23H22F2N6O/c1-32-16-11-9-14(10-12-16)27-22-26-13-19-21(30-22)31(15-5-2-3-6-15)23(28-19)29-20-17(24)7-4-8-18(20)25/h4,7-13,15H,2-3,5-6H2,1H3,(H,28,29)(H,26,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of full length Hexa-His-tagged JNK2 using GST-tagged cJun and [gamma33P]ATP as substrate after 60 mins by scintillation counting |
Bioorg Med Chem Lett 22: 1427-32 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.028
BindingDB Entry DOI: 10.7270/Q2513ZP6 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50288275

(3-(4-Isobutyl-2-methyl-phenyl)-thiophene-2-sulfoni...)Show SMILES CC(C)Cc1ccc(-c2ccsc2S(=O)(=O)Nc2onc(C)c2Br)c(C)c1 Show InChI InChI=1S/C19H21BrN2O3S2/c1-11(2)9-14-5-6-15(12(3)10-14)16-7-8-26-19(16)27(23,24)22-18-17(20)13(4)21-25-18/h5-8,10-11,22H,9H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for binding affinity against Endothelin A receptor |
Bioorg Med Chem Lett 6: 2651-2656 (1996)
Article DOI: 10.1016/S0960-894X(96)00496-9
BindingDB Entry DOI: 10.7270/Q24M94HV |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50363454

(CHEMBL1946332)Show InChI InChI=1S/C22H27FN6/c23-17-12-6-7-13-18(17)26-22-27-19-14-24-21(25-15-8-2-1-3-9-15)28-20(19)29(22)16-10-4-5-11-16/h6-7,12-16H,1-5,8-11H2,(H,26,27)(H,24,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of full length Hexa-His-tagged JNK3 using GST-tagged cJun and [gamma33P]ATP as substrate after 60 mins by scintillation counting |
Bioorg Med Chem Lett 22: 1427-32 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.028
BindingDB Entry DOI: 10.7270/Q2513ZP6 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50363463
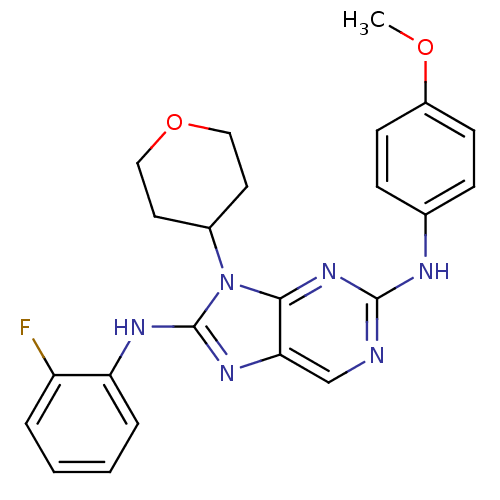
(CHEMBL1946341)Show SMILES COc1ccc(Nc2ncc3nc(Nc4ccccc4F)n(C4CCOCC4)c3n2)cc1 Show InChI InChI=1S/C23H23FN6O2/c1-31-17-8-6-15(7-9-17)26-22-25-14-20-21(29-22)30(16-10-12-32-13-11-16)23(28-20)27-19-5-3-2-4-18(19)24/h2-9,14,16H,10-13H2,1H3,(H,27,28)(H,25,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of full length Hexa-His-tagged JNK2 using GST-tagged cJun and [gamma33P]ATP as substrate after 60 mins by scintillation counting |
Bioorg Med Chem Lett 22: 1427-32 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.028
BindingDB Entry DOI: 10.7270/Q2513ZP6 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50288002

(5-Bromo-N-(3,4-dimethyl-isoxazol-5-yl)-2-ethyl-ben...)Show InChI InChI=1S/C13H15BrN2O3S/c1-4-10-5-6-11(14)7-12(10)20(17,18)16-13-8(2)9(3)15-19-13/h5-7,16H,4H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against human Endothelin A receptor in TE 671(ATCC# HTB 139) cell membrane preparation |
Bioorg Med Chem Lett 6: 2393-2398 (1996)
Article DOI: 10.1016/0960-894X(96)00441-6
BindingDB Entry DOI: 10.7270/Q22V2G3R |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50288014

(CHEMBL311461 | N-(4-Bromo-3-methyl-isoxazol-5-yl)-...)Show InChI InChI=1S/C10H7BrCl2N2O3S/c1-5-9(11)10(18-14-5)15-19(16,17)8-3-6(12)2-7(13)4-8/h2-4,15H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against human Endothelin A receptor in TE 671(ATCC# HTB 139) cell membrane preparation |
Bioorg Med Chem Lett 6: 2393-2398 (1996)
Article DOI: 10.1016/0960-894X(96)00441-6
BindingDB Entry DOI: 10.7270/Q22V2G3R |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50363463

(CHEMBL1946341)Show SMILES COc1ccc(Nc2ncc3nc(Nc4ccccc4F)n(C4CCOCC4)c3n2)cc1 Show InChI InChI=1S/C23H23FN6O2/c1-31-17-8-6-15(7-9-17)26-22-25-14-20-21(29-22)30(16-10-12-32-13-11-16)23(28-20)27-19-5-3-2-4-18(19)24/h2-9,14,16H,10-13H2,1H3,(H,27,28)(H,25,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of full length Hexa-His-tagged JNK3 using GST-tagged cJun and [gamma33P]ATP as substrate after 60 mins by scintillation counting |
Bioorg Med Chem Lett 22: 1427-32 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.028
BindingDB Entry DOI: 10.7270/Q2513ZP6 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50363453
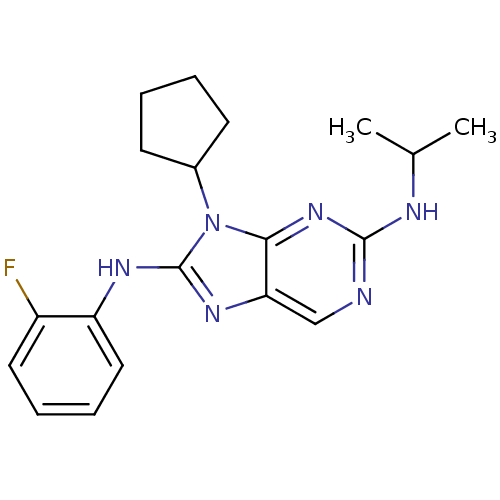
(CHEMBL1946331)Show InChI InChI=1S/C19H23FN6/c1-12(2)22-18-21-11-16-17(25-18)26(13-7-3-4-8-13)19(24-16)23-15-10-6-5-9-14(15)20/h5-6,9-13H,3-4,7-8H2,1-2H3,(H,23,24)(H,21,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of full length Hexa-His-tagged JNK2 using GST-tagged cJun and [gamma33P]ATP as substrate after 60 mins by scintillation counting |
Bioorg Med Chem Lett 22: 1427-32 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.028
BindingDB Entry DOI: 10.7270/Q2513ZP6 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50288042
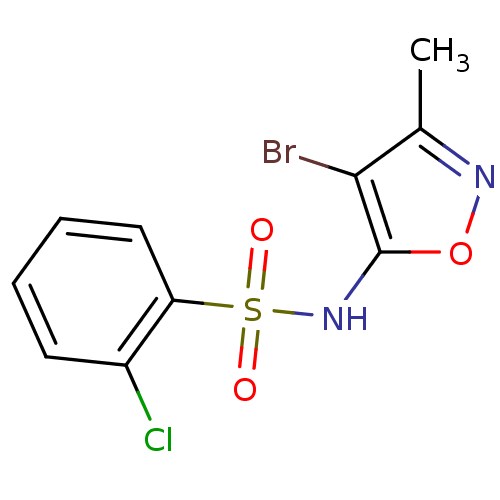
(CHEMBL80438 | N-(4-Bromo-3-methyl-isoxazol-5-yl)-2...)Show InChI InChI=1S/C10H8BrClN2O3S/c1-6-9(11)10(17-13-6)14-18(15,16)8-5-3-2-4-7(8)12/h2-5,14H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against human Endothelin A receptor in TE 671(ATCC# HTB 139) cell membrane preparation |
Bioorg Med Chem Lett 6: 2393-2398 (1996)
Article DOI: 10.1016/0960-894X(96)00441-6
BindingDB Entry DOI: 10.7270/Q22V2G3R |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50288011
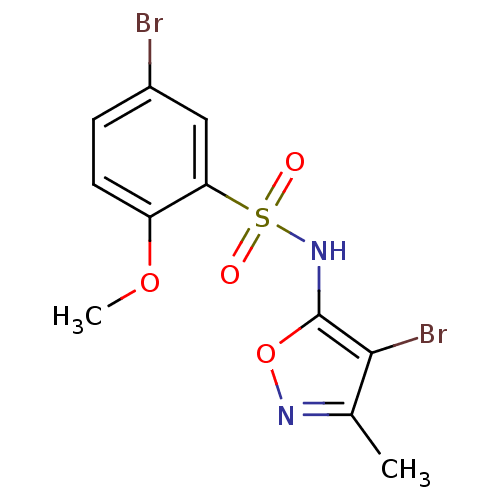
(5-Bromo-N-(4-bromo-3-methyl-isoxazol-5-yl)-2-metho...)Show InChI InChI=1S/C11H10Br2N2O4S/c1-6-10(13)11(19-14-6)15-20(16,17)9-5-7(12)3-4-8(9)18-2/h3-5,15H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against human Endothelin A receptor in TE 671(ATCC# HTB 139) cell membrane preparation |
Bioorg Med Chem Lett 6: 2393-2398 (1996)
Article DOI: 10.1016/0960-894X(96)00441-6
BindingDB Entry DOI: 10.7270/Q22V2G3R |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50363455

(CHEMBL1946333)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1ncc2nc(Nc3ccccc3F)n(C3CCCC3)c2n1 |r,wU:1.0,wD:4.7,(-11.53,-21.51,;-10.75,-20.18,;-11.51,-18.84,;-10.74,-17.51,;-9.2,-17.52,;-8.43,-18.84,;-9.2,-20.18,;-8.44,-16.18,;-6.9,-16.18,;-6.13,-14.85,;-4.59,-14.85,;-3.83,-16.18,;-2.33,-16.49,;-2.17,-18.02,;-.84,-18.79,;.49,-18.03,;1.83,-18.8,;3.16,-18.02,;3.16,-16.47,;1.82,-15.71,;.5,-16.48,;-.84,-15.71,;-3.58,-18.65,;-3.58,-20.19,;-4.83,-21.09,;-4.36,-22.55,;-2.82,-22.56,;-2.34,-21.1,;-4.6,-17.51,;-6.13,-17.51,)| Show InChI InChI=1S/C22H27FN6O/c23-17-7-3-4-8-18(17)26-22-27-19-13-24-21(25-14-9-11-16(30)12-10-14)28-20(19)29(22)15-5-1-2-6-15/h3-4,7-8,13-16,30H,1-2,5-6,9-12H2,(H,26,27)(H,24,25,28)/t14-,16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of full length Hexa-His-tagged JNK1 using GST-tagged cJun and [gamma33P]ATP as substrate after 60 mins by scintillation counting |
Bioorg Med Chem Lett 22: 1427-32 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.028
BindingDB Entry DOI: 10.7270/Q2513ZP6 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50288024

(CHEMBL78080 | N-(4-Bromo-3-methyl-isoxazol-5-yl)-b...)Show InChI InChI=1S/C10H9BrN2O3S/c1-7-9(11)10(16-12-7)13-17(14,15)8-5-3-2-4-6-8/h2-6,13H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against human Endothelin A receptor in TE 671(ATCC# HTB 139) cell membrane preparation |
Bioorg Med Chem Lett 6: 2393-2398 (1996)
Article DOI: 10.1016/0960-894X(96)00441-6
BindingDB Entry DOI: 10.7270/Q22V2G3R |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50288280

(3-(4-Isobutyl-phenyl)-thiophene-2-sulfonic acid (4...)Show SMILES CC(C)Cc1ccc(cc1)-c1ccsc1S(=O)(=O)Nc1onc(C)c1Br Show InChI InChI=1S/C18H19BrN2O3S2/c1-11(2)10-13-4-6-14(7-5-13)15-8-9-25-18(15)26(22,23)21-17-16(19)12(3)20-24-17/h4-9,11,21H,10H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for binding affinity against Endothelin A receptor |
Bioorg Med Chem Lett 6: 2651-2656 (1996)
Article DOI: 10.1016/S0960-894X(96)00496-9
BindingDB Entry DOI: 10.7270/Q24M94HV |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50363458
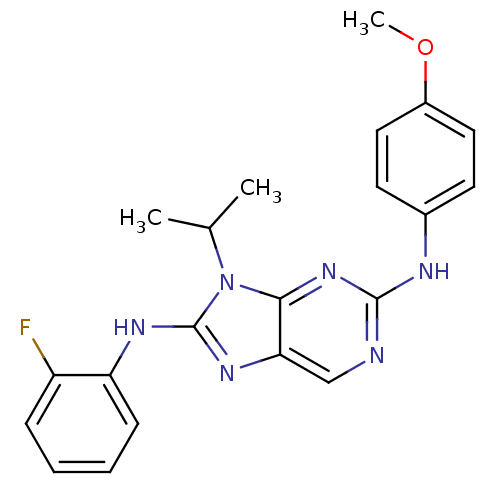
(CHEMBL1946336)Show SMILES COc1ccc(Nc2ncc3nc(Nc4ccccc4F)n(C(C)C)c3n2)cc1 Show InChI InChI=1S/C21H21FN6O/c1-13(2)28-19-18(26-21(28)25-17-7-5-4-6-16(17)22)12-23-20(27-19)24-14-8-10-15(29-3)11-9-14/h4-13H,1-3H3,(H,25,26)(H,23,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of full length Hexa-His-tagged JNK2 using GST-tagged cJun and [gamma33P]ATP as substrate after 60 mins by scintillation counting |
Bioorg Med Chem Lett 22: 1427-32 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.028
BindingDB Entry DOI: 10.7270/Q2513ZP6 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50288277

(3-(4-Propyl-phenyl)-thiophene-2-sulfonic acid (4-b...)Show SMILES CCCc1ccc(cc1)-c1ccsc1S(=O)(=O)Nc1onc(C)c1Br Show InChI InChI=1S/C17H17BrN2O3S2/c1-3-4-12-5-7-13(8-6-12)14-9-10-24-17(14)25(21,22)20-16-15(18)11(2)19-23-16/h5-10,20H,3-4H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for binding affinity against Endothelin A receptor |
Bioorg Med Chem Lett 6: 2651-2656 (1996)
Article DOI: 10.1016/S0960-894X(96)00496-9
BindingDB Entry DOI: 10.7270/Q24M94HV |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50363465

(CHEMBL1946646)Show SMILES N[C@H]1CC[C@@H](CC1)Nc1ncc2nc(Nc3c(F)cccc3F)n(C3CCCC3)c2n1 |r,wU:4.7,wD:1.0,(-8.05,-2.1,;-7.28,-.77,;-5.74,-.77,;-4.96,.56,;-5.74,1.89,;-7.27,1.89,;-8.04,.57,;-4.97,3.22,;-3.43,3.22,;-2.66,4.56,;-1.12,4.55,;-.36,3.22,;1.14,2.91,;1.29,1.38,;2.63,.61,;3.96,1.38,;3.96,2.92,;2.63,3.69,;5.29,3.7,;6.63,2.93,;6.63,1.38,;5.3,.61,;5.3,-.93,;-.11,.75,;-.11,-.78,;-1.36,-1.68,;-.89,-3.15,;.65,-3.15,;1.13,-1.69,;-1.14,1.9,;-2.66,1.9,)| Show InChI InChI=1S/C22H27F2N7/c23-16-6-3-7-17(24)19(16)29-22-28-18-12-26-21(27-14-10-8-13(25)9-11-14)30-20(18)31(22)15-4-1-2-5-15/h3,6-7,12-15H,1-2,4-5,8-11,25H2,(H,28,29)(H,26,27,30)/t13-,14- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of full length Hexa-His-tagged JNK1 using GST-tagged cJun and [gamma33P]ATP as substrate after 60 mins by scintillation counting |
Bioorg Med Chem Lett 22: 1427-32 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.028
BindingDB Entry DOI: 10.7270/Q2513ZP6 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50363458

(CHEMBL1946336)Show SMILES COc1ccc(Nc2ncc3nc(Nc4ccccc4F)n(C(C)C)c3n2)cc1 Show InChI InChI=1S/C21H21FN6O/c1-13(2)28-19-18(26-21(28)25-17-7-5-4-6-16(17)22)12-23-20(27-19)24-14-8-10-15(29-3)11-9-14/h4-13H,1-3H3,(H,25,26)(H,23,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of full length Hexa-His-tagged JNK3 using GST-tagged cJun and [gamma33P]ATP as substrate after 60 mins by scintillation counting |
Bioorg Med Chem Lett 22: 1427-32 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.028
BindingDB Entry DOI: 10.7270/Q2513ZP6 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50288263

(3-(2,4-Dimethyl-phenyl)-thiophene-2-sulfonic acid ...)Show InChI InChI=1S/C16H15BrN2O3S2/c1-9-4-5-12(10(2)8-9)13-6-7-23-16(13)24(20,21)19-15-14(17)11(3)18-22-15/h4-8,19H,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for binding affinity against Endothelin A receptor |
Bioorg Med Chem Lett 6: 2651-2656 (1996)
Article DOI: 10.1016/S0960-894X(96)00496-9
BindingDB Entry DOI: 10.7270/Q24M94HV |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50288004

(CHEMBL309397 | N-(4-Bromo-3-methyl-isoxazol-5-yl)-...)Show InChI InChI=1S/C10H7BrCl2N2O3S/c1-5-9(11)10(18-14-5)15-19(16,17)8-4-6(12)2-3-7(8)13/h2-4,15H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against human Endothelin A receptor in TE 671(ATCC# HTB 139) cell membrane preparation |
Bioorg Med Chem Lett 6: 2393-2398 (1996)
Article DOI: 10.1016/0960-894X(96)00441-6
BindingDB Entry DOI: 10.7270/Q22V2G3R |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50363453

(CHEMBL1946331)Show InChI InChI=1S/C19H23FN6/c1-12(2)22-18-21-11-16-17(25-18)26(13-7-3-4-8-13)19(24-16)23-15-10-6-5-9-14(15)20/h5-6,9-13H,3-4,7-8H2,1-2H3,(H,23,24)(H,21,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of full length Hexa-His-tagged JNK3 using GST-tagged cJun and [gamma33P]ATP as substrate after 60 mins by scintillation counting |
Bioorg Med Chem Lett 22: 1427-32 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.028
BindingDB Entry DOI: 10.7270/Q2513ZP6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data