 Found 43 hits with Last Name = 'lopes' and Initial = 'a'
Found 43 hits with Last Name = 'lopes' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50590814
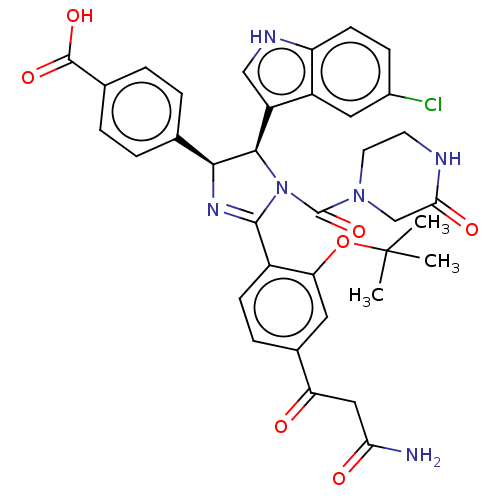
(CHEMBL5182359)Show SMILES CC(C)(C)Oc1cc(ccc1C1=N[C@H]([C@H](N1C(=O)N1CCNC(=O)C1)c1c[nH]c2ccc(Cl)cc12)c1ccc(cc1)C(O)=O)C(=O)CC(N)=O |r,t:12| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114637
BindingDB Entry DOI: 10.7270/Q29C72CM |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50590815
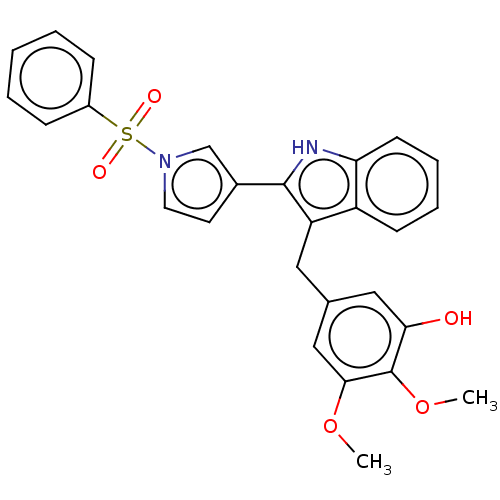
(CHEMBL5199785)Show SMILES COc1cc(Cc2c([nH]c3ccccc23)-c2ccn(c2)S(=O)(=O)c2ccccc2)cc(O)c1OC | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114637
BindingDB Entry DOI: 10.7270/Q29C72CM |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50590813

(CHEMBL5204846)Show SMILES Cc1c(Cl)ccc2cc(\C=C3/NC(=O)N(C(C(=O)NC(CO)CO)c4ccc(F)c(F)c4)C3=O)[nH]c12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114637
BindingDB Entry DOI: 10.7270/Q29C72CM |
More data for this
Ligand-Target Pair | |
Protein Mdm4
(Homo sapiens (Human)) | BDBM50590815

(CHEMBL5199785)Show SMILES COc1cc(Cc2c([nH]c3ccccc23)-c2ccn(c2)S(=O)(=O)c2ccccc2)cc(O)c1OC | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114637
BindingDB Entry DOI: 10.7270/Q29C72CM |
More data for this
Ligand-Target Pair | |
Protein Mdm4
(Homo sapiens (Human)) | BDBM50590813

(CHEMBL5204846)Show SMILES Cc1c(Cl)ccc2cc(\C=C3/NC(=O)N(C(C(=O)NC(CO)CO)c4ccc(F)c(F)c4)C3=O)[nH]c12 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114637
BindingDB Entry DOI: 10.7270/Q29C72CM |
More data for this
Ligand-Target Pair | |
Protein Mdm4
(Homo sapiens (Human)) | BDBM50590814

(CHEMBL5182359)Show SMILES CC(C)(C)Oc1cc(ccc1C1=N[C@H]([C@H](N1C(=O)N1CCNC(=O)C1)c1c[nH]c2ccc(Cl)cc12)c1ccc(cc1)C(O)=O)C(=O)CC(N)=O |r,t:12| | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114637
BindingDB Entry DOI: 10.7270/Q29C72CM |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk2
(Homo sapiens (Human)) | BDBM50059889

((staurosporine)3-methoxy-2-methyl-4-methylamino-(2...)Show SMILES CN[C@@H]1CC2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20?,26-,28+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro
Curated by ChEMBL
| Assay Description
Inhibition of human CHK2 kinase expressed in insect Sf21 cells using phospho-CREBtide as substrate at 30 uM |
Eur J Med Chem 46: 1245-53 (2011)
Article DOI: 10.1016/j.ejmech.2011.01.045
BindingDB Entry DOI: 10.7270/Q27M08ZV |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50396783
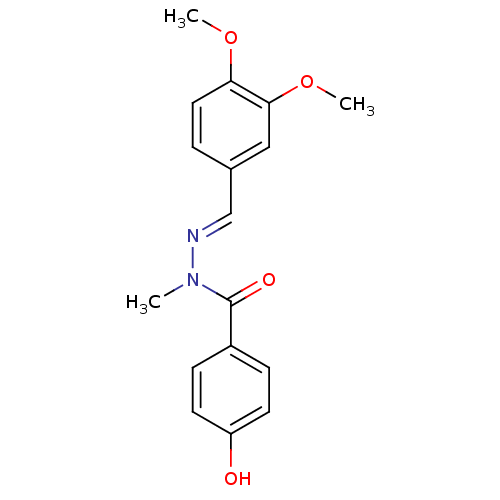
(CHEMBL2172710)Show InChI InChI=1S/C17H18N2O4/c1-19(17(21)13-5-7-14(20)8-6-13)18-11-12-4-9-15(22-2)16(10-12)23-3/h4-11,20H,1-3H3/b18-11+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4D |
J Med Chem 55: 7525-45 (2012)
Article DOI: 10.1021/jm300514y
BindingDB Entry DOI: 10.7270/Q2CC11TC |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit alpha
(Homo sapiens (Human)) | BDBM50059889

((staurosporine)3-methoxy-2-methyl-4-methylamino-(2...)Show SMILES CN[C@@H]1CC2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20?,26-,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IKK-alpha expressed in insect Sf21 cells using phospho-Ulight-IkappaB-alpha as substrate at 30 uM |
Eur J Med Chem 46: 1245-53 (2011)
Article DOI: 10.1016/j.ejmech.2011.01.045
BindingDB Entry DOI: 10.7270/Q27M08ZV |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50396783

(CHEMBL2172710)Show InChI InChI=1S/C17H18N2O4/c1-19(17(21)13-5-7-14(20)8-6-13)18-11-12-4-9-15(22-2)16(10-12)23-3/h4-11,20H,1-3H3/b18-11+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B |
J Med Chem 55: 7525-45 (2012)
Article DOI: 10.1021/jm300514y
BindingDB Entry DOI: 10.7270/Q2CC11TC |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50398447
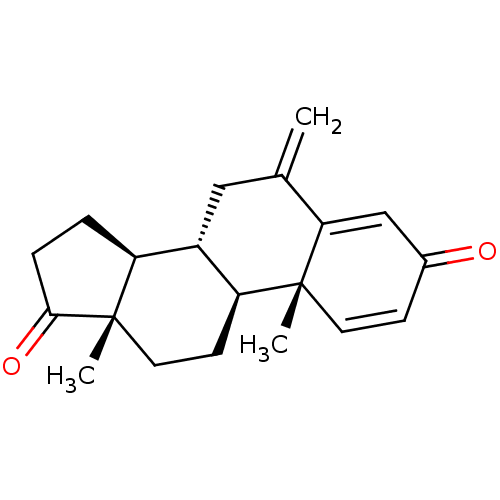
(Aromasin | EXEMESTANE)Show SMILES C[C@]12CC[C@H]3[C@@H](CC(=C)C4=CC(=O)C=C[C@]34C)[C@@H]1CCC2=O |r,c:13,t:9| Show InChI InChI=1S/C20H24O2/c1-12-10-14-15-4-5-18(22)20(15,3)9-7-16(14)19(2)8-6-13(21)11-17(12)19/h6,8,11,14-16H,1,4-5,7,9-10H2,2-3H3/t14-,15-,16-,19+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra
Curated by ChEMBL
| Assay Description
Inhibition of human placental microsome aromatase using [1beta-3H] androstenedione as substrate after 15 mins by liquid scintillation counting |
Eur J Med Chem 87: 336-45 (2014)
Article DOI: 10.1016/j.ejmech.2014.09.074
BindingDB Entry DOI: 10.7270/Q2JH3NWD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50396784
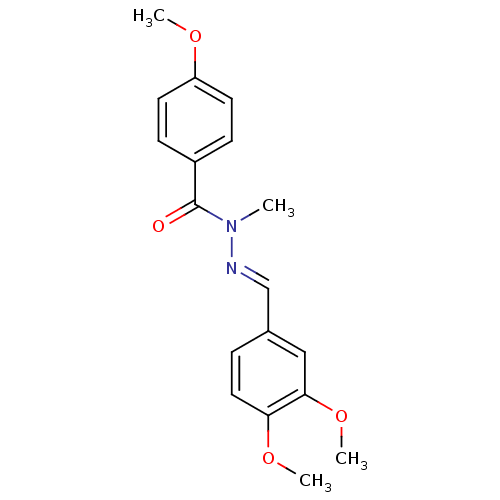
(CHEMBL2172734)Show InChI InChI=1S/C18H20N2O4/c1-20(18(21)14-6-8-15(22-2)9-7-14)19-12-13-5-10-16(23-3)17(11-13)24-4/h5-12H,1-4H3/b19-12+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B |
J Med Chem 55: 7525-45 (2012)
Article DOI: 10.1021/jm300514y
BindingDB Entry DOI: 10.7270/Q2CC11TC |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50396783

(CHEMBL2172710)Show InChI InChI=1S/C17H18N2O4/c1-19(17(21)13-5-7-14(20)8-6-13)18-11-12-4-9-15(22-2)16(10-12)23-3/h4-11,20H,1-3H3/b18-11+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4A |
J Med Chem 55: 7525-45 (2012)
Article DOI: 10.1021/jm300514y
BindingDB Entry DOI: 10.7270/Q2CC11TC |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50396784

(CHEMBL2172734)Show InChI InChI=1S/C18H20N2O4/c1-20(18(21)14-6-8-15(22-2)9-7-14)19-12-13-5-10-16(23-3)17(11-13)24-4/h5-12H,1-4H3/b19-12+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4D |
J Med Chem 55: 7525-45 (2012)
Article DOI: 10.1021/jm300514y
BindingDB Entry DOI: 10.7270/Q2CC11TC |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50069753

(CHEMBL3407538)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC(=C)C2=CC(=O)C=C[C@]12C |r,c:23,t:19| Show InChI InChI=1S/C20H26O2/c1-12-10-14-15-4-5-18(22)20(15,3)9-7-16(14)19(2)8-6-13(21)11-17(12)19/h6,8,11,14-16,18,22H,1,4-5,7,9-10H2,2-3H3/t14-,15-,16-,18-,19+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra
Curated by ChEMBL
| Assay Description
Inhibition of human placental microsome aromatase using [1beta-3H] androstenedione as substrate after 15 mins by liquid scintillation counting |
Eur J Med Chem 87: 336-45 (2014)
Article DOI: 10.1016/j.ejmech.2014.09.074
BindingDB Entry DOI: 10.7270/Q2JH3NWD |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50396782
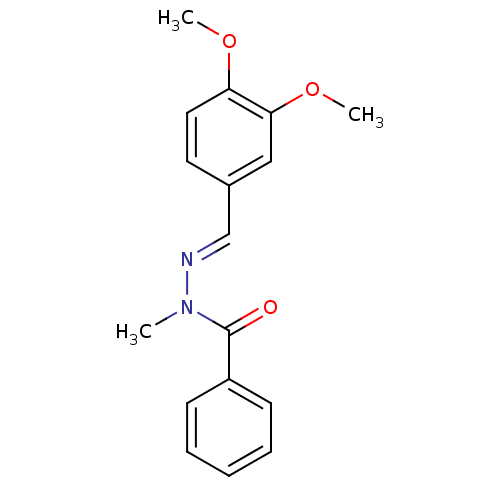
(CHEMBL2172731)Show InChI InChI=1S/C17H18N2O3/c1-19(17(20)14-7-5-4-6-8-14)18-12-13-9-10-15(21-2)16(11-13)22-3/h4-12H,1-3H3/b18-12+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4D |
J Med Chem 55: 7525-45 (2012)
Article DOI: 10.1021/jm300514y
BindingDB Entry DOI: 10.7270/Q2CC11TC |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50396784

(CHEMBL2172734)Show InChI InChI=1S/C18H20N2O4/c1-20(18(21)14-6-8-15(22-2)9-7-14)19-12-13-5-10-16(23-3)17(11-13)24-4/h5-12H,1-4H3/b19-12+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 159 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4A |
J Med Chem 55: 7525-45 (2012)
Article DOI: 10.1021/jm300514y
BindingDB Entry DOI: 10.7270/Q2CC11TC |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50396785

(CHEMBL2172707)Show InChI InChI=1S/C8H5ClN2/c9-8-6-3-1-2-4-7(6)10-5-11-8/h1-5H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 162 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4A |
J Med Chem 55: 7525-45 (2012)
Article DOI: 10.1021/jm300514y
BindingDB Entry DOI: 10.7270/Q2CC11TC |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM14361

((R,S)-Rolipram | 4-(3-cyclopentyloxy-4-methoxy-phe...)Show InChI InChI=1S/C16H21NO3/c1-19-14-7-6-11(12-9-16(18)17-10-12)8-15(14)20-13-4-2-3-5-13/h6-8,12-13H,2-5,9-10H2,1H3,(H,17,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 162 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4A |
J Med Chem 55: 7525-45 (2012)
Article DOI: 10.1021/jm300514y
BindingDB Entry DOI: 10.7270/Q2CC11TC |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Homo sapiens (Human)) | BDBM50396783

(CHEMBL2172710)Show InChI InChI=1S/C17H18N2O4/c1-19(17(21)13-5-7-14(20)8-6-13)18-11-12-4-9-15(22-2)16(10-12)23-3/h4-11,20H,1-3H3/b18-11+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 206 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4C |
J Med Chem 55: 7525-45 (2012)
Article DOI: 10.1021/jm300514y
BindingDB Entry DOI: 10.7270/Q2CC11TC |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50396785

(CHEMBL2172707)Show InChI InChI=1S/C8H5ClN2/c9-8-6-3-1-2-4-7(6)10-5-11-8/h1-5H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 231 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B |
J Med Chem 55: 7525-45 (2012)
Article DOI: 10.1021/jm300514y
BindingDB Entry DOI: 10.7270/Q2CC11TC |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM14361

((R,S)-Rolipram | 4-(3-cyclopentyloxy-4-methoxy-phe...)Show InChI InChI=1S/C16H21NO3/c1-19-14-7-6-11(12-9-16(18)17-10-12)8-15(14)20-13-4-2-3-5-13/h6-8,12-13H,2-5,9-10H2,1H3,(H,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 231 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B |
J Med Chem 55: 7525-45 (2012)
Article DOI: 10.1021/jm300514y
BindingDB Entry DOI: 10.7270/Q2CC11TC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aromatase
(Homo sapiens (Human)) | BDBM50069753

(CHEMBL3407538)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC(=C)C2=CC(=O)C=C[C@]12C |r,c:23,t:19| Show InChI InChI=1S/C20H26O2/c1-12-10-14-15-4-5-18(22)20(15,3)9-7-16(14)19(2)8-6-13(21)11-17(12)19/h6,8,11,14-16,18,22H,1,4-5,7,9-10H2,2-3H3/t14-,15-,16-,18-,19+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra
Curated by ChEMBL
| Assay Description
Inhibition of human placental microsome aromatase expressed in human MCF7 cells using [1beta-3H] androstenedione as substrate after 1 hr by liquid sc... |
Eur J Med Chem 87: 336-45 (2014)
Article DOI: 10.1016/j.ejmech.2014.09.074
BindingDB Entry DOI: 10.7270/Q2JH3NWD |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50059889

((staurosporine)3-methoxy-2-methyl-4-methylamino-(2...)Show SMILES CN[C@@H]1CC2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20?,26-,28+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IKK-beta expressed in insect Sf21 cells using phospho-Ulight-IkappaB-alpha as substrate at 30 uM |
Eur J Med Chem 46: 1245-53 (2011)
Article DOI: 10.1016/j.ejmech.2011.01.045
BindingDB Entry DOI: 10.7270/Q27M08ZV |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Homo sapiens (Human)) | BDBM50396784

(CHEMBL2172734)Show InChI InChI=1S/C18H20N2O4/c1-20(18(21)14-6-8-15(22-2)9-7-14)19-12-13-5-10-16(23-3)17(11-13)24-4/h5-12H,1-4H3/b19-12+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 505 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4C |
J Med Chem 55: 7525-45 (2012)
Article DOI: 10.1021/jm300514y
BindingDB Entry DOI: 10.7270/Q2CC11TC |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50069755
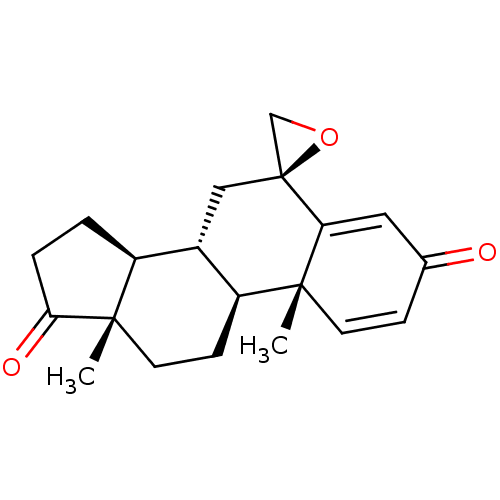
(CHEMBL3407536)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])C[C@]2(CO2)C2=CC(=O)C=C[C@]12C |r,c:25,t:21| Show InChI InChI=1S/C20H24O3/c1-18-7-5-12(21)9-16(18)20(11-23-20)10-13-14-3-4-17(22)19(14,2)8-6-15(13)18/h5,7,9,13-15H,3-4,6,8,10-11H2,1-2H3/t13-,14-,15-,18+,19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra
Curated by ChEMBL
| Assay Description
Inhibition of human placental microsome aromatase using [1beta-3H] androstenedione as substrate after 15 mins by liquid scintillation counting |
Eur J Med Chem 87: 336-45 (2014)
Article DOI: 10.1016/j.ejmech.2014.09.074
BindingDB Entry DOI: 10.7270/Q2JH3NWD |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50396785

(CHEMBL2172707)Show InChI InChI=1S/C8H5ClN2/c9-8-6-3-1-2-4-7(6)10-5-11-8/h1-5H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 622 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4D |
J Med Chem 55: 7525-45 (2012)
Article DOI: 10.1021/jm300514y
BindingDB Entry DOI: 10.7270/Q2CC11TC |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM14361

((R,S)-Rolipram | 4-(3-cyclopentyloxy-4-methoxy-phe...)Show InChI InChI=1S/C16H21NO3/c1-19-14-7-6-11(12-9-16(18)17-10-12)8-15(14)20-13-4-2-3-5-13/h6-8,12-13H,2-5,9-10H2,1H3,(H,17,18) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 622 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4D |
J Med Chem 55: 7525-45 (2012)
Article DOI: 10.1021/jm300514y
BindingDB Entry DOI: 10.7270/Q2CC11TC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aromatase
(Homo sapiens (Human)) | BDBM50069752
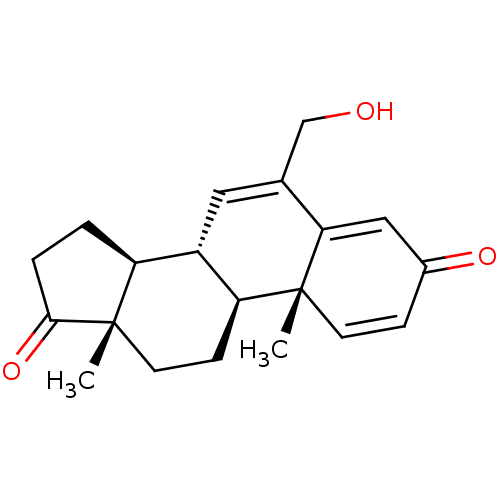
(CHEMBL3407539)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])C=C(CO)C2=CC(=O)C=C[C@]12C |r,c:24,t:16,20| Show InChI InChI=1S/C20H24O3/c1-19-7-5-13(22)10-17(19)12(11-21)9-14-15-3-4-18(23)20(15,2)8-6-16(14)19/h5,7,9-10,14-16,21H,3-4,6,8,11H2,1-2H3/t14-,15-,16-,19+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra
Curated by ChEMBL
| Assay Description
Inhibition of human placental microsome aromatase using [1beta-3H] androstenedione as substrate after 15 mins by liquid scintillation counting |
Eur J Med Chem 87: 336-45 (2014)
Article DOI: 10.1016/j.ejmech.2014.09.074
BindingDB Entry DOI: 10.7270/Q2JH3NWD |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50069755

(CHEMBL3407536)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])C[C@]2(CO2)C2=CC(=O)C=C[C@]12C |r,c:25,t:21| Show InChI InChI=1S/C20H24O3/c1-18-7-5-12(21)9-16(18)20(11-23-20)10-13-14-3-4-17(22)19(14,2)8-6-15(13)18/h5,7,9,13-15H,3-4,6,8,10-11H2,1-2H3/t13-,14-,15-,18+,19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra
Curated by ChEMBL
| Assay Description
Inhibition of human placental microsome aromatase expressed in human MCF7 cells using [1beta-3H] androstenedione as substrate after 1 hr by liquid sc... |
Eur J Med Chem 87: 336-45 (2014)
Article DOI: 10.1016/j.ejmech.2014.09.074
BindingDB Entry DOI: 10.7270/Q2JH3NWD |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM14769

(6-(3,4-Dimethoxy-phenyl)-4,5-dimethyl-4,5-dihydro-...)Show InChI InChI=1S/C12H10F2N2O3/c1-18-10-6-7(2-4-9(10)19-12(13)14)8-3-5-11(17)16-15-8/h2-6,12H,1H3,(H,16,17) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4D |
J Med Chem 55: 7525-45 (2012)
Article DOI: 10.1021/jm300514y
BindingDB Entry DOI: 10.7270/Q2CC11TC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aromatase
(Homo sapiens (Human)) | BDBM50069754
(CHEMBL3407537)Show SMILES [H][C@@]12O[C@]1([H])[C@@]1(C)C(=CC2=O)C(=C)C[C@@]2([H])[C@]3([H])CCC(=O)[C@@]3(C)CC[C@]12[H] |r,c:8| Show InChI InChI=1S/C20H24O3/c1-10-8-11-12-4-5-16(22)19(12,2)7-6-13(11)20(3)14(10)9-15(21)17-18(20)23-17/h9,11-13,17-18H,1,4-8H2,2-3H3/t11-,12-,13-,17-,18-,19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra
Curated by ChEMBL
| Assay Description
Inhibition of human placental microsome aromatase using [1beta-3H] androstenedione as substrate after 15 mins by liquid scintillation counting |
Eur J Med Chem 87: 336-45 (2014)
Article DOI: 10.1016/j.ejmech.2014.09.074
BindingDB Entry DOI: 10.7270/Q2JH3NWD |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50398447

(Aromasin | EXEMESTANE)Show SMILES C[C@]12CC[C@H]3[C@@H](CC(=C)C4=CC(=O)C=C[C@]34C)[C@@H]1CCC2=O |r,c:13,t:9| Show InChI InChI=1S/C20H24O2/c1-12-10-14-15-4-5-18(22)20(15,3)9-7-16(14)19(2)8-6-13(21)11-17(12)19/h6,8,11,14-16H,1,4-5,7,9-10H2,2-3H3/t14-,15-,16-,19+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra
Curated by ChEMBL
| Assay Description
Inhibition of human placental microsome aromatase expressed in human MCF7 cells using [1beta-3H] androstenedione as substrate after 1 hr by liquid sc... |
Eur J Med Chem 87: 336-45 (2014)
Article DOI: 10.1016/j.ejmech.2014.09.074
BindingDB Entry DOI: 10.7270/Q2JH3NWD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aromatase
(Homo sapiens (Human)) | BDBM50069752

(CHEMBL3407539)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])C=C(CO)C2=CC(=O)C=C[C@]12C |r,c:24,t:16,20| Show InChI InChI=1S/C20H24O3/c1-19-7-5-13(22)10-17(19)12(11-21)9-14-15-3-4-18(23)20(15,2)8-6-16(14)19/h5,7,9-10,14-16,21H,3-4,6,8,11H2,1-2H3/t14-,15-,16-,19+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra
Curated by ChEMBL
| Assay Description
Inhibition of human placental microsome aromatase expressed in human MCF7 cells using [1beta-3H] androstenedione as substrate after 1 hr by liquid sc... |
Eur J Med Chem 87: 336-45 (2014)
Article DOI: 10.1016/j.ejmech.2014.09.074
BindingDB Entry DOI: 10.7270/Q2JH3NWD |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50069754

(CHEMBL3407537)Show SMILES [H][C@@]12O[C@]1([H])[C@@]1(C)C(=CC2=O)C(=C)C[C@@]2([H])[C@]3([H])CCC(=O)[C@@]3(C)CC[C@]12[H] |r,c:8| Show InChI InChI=1S/C20H24O3/c1-10-8-11-12-4-5-16(22)19(12,2)7-6-13(11)20(3)14(10)9-15(21)17-18(20)23-17/h9,11-13,17-18H,1,4-8H2,2-3H3/t11-,12-,13-,17-,18-,19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra
Curated by ChEMBL
| Assay Description
Inhibition of human placental microsome aromatase expressed in human MCF7 cells using [1beta-3H] androstenedione as substrate after 1 hr by liquid sc... |
Eur J Med Chem 87: 336-45 (2014)
Article DOI: 10.1016/j.ejmech.2014.09.074
BindingDB Entry DOI: 10.7270/Q2JH3NWD |
More data for this
Ligand-Target Pair | |
Transthyretin
(Homo sapiens (Human)) | BDBM408979

(US10377729, Compound 2OH-PCB80)Show SMILES [O-]c1c(Cl)cc(cc1Cl)-c1cc(Cl)c([O-])c(Cl)c1 Show InChI InChI=1S/C12H6Cl4O2/c13-7-1-5(2-8(14)11(7)17)6-3-9(15)12(18)10(16)4-6/h1-4,17-18H/p-2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
BSIM Therapeutics, S.A.
US Patent
| Assay Description
In vitro evaluation of inhibitory activity against WT-TTR amyloid fibril formation of AT09 and reference compounds. Reference compounds (thyroxine, t... |
US Patent US10377729 (2019)
BindingDB Entry DOI: 10.7270/Q2WM1GSC |
More data for this
Ligand-Target Pair | |
Transthyretin
(Homo sapiens (Human)) | BDBM408976

(US10377729, Compound AT09-B00)Show InChI InChI=1S/C12H9ClO4/c1-7-8(12(14)15)6-17-10(7)2-3-11-9(13)4-5-16-11/h2-6H,1H3,(H,14,15)/p-1/b3-2+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
BSIM Therapeutics, S.A.
US Patent
| Assay Description
In vitro evaluation of inhibitory activity against WT-TTR amyloid fibril formation of AT09 and reference compounds. Reference compounds (thyroxine, t... |
US Patent US10377729 (2019)
BindingDB Entry DOI: 10.7270/Q2WM1GSC |
More data for this
Ligand-Target Pair | |
Transthyretin
(Homo sapiens (Human)) | BDBM408980
(Thyroxine | US10377729, T4)Show SMILES [NH3+][C@@H](Cc1cc(I)c(Oc2cc(I)c(O)c(I)c2)c(I)c1)C([O-])=O |r| Show InChI InChI=1S/C15H11I4NO4/c16-8-4-7(5-9(17)13(8)21)24-14-10(18)1-6(2-11(14)19)3-12(20)15(22)23/h1-2,4-5,12,21H,3,20H2,(H,22,23)/t12-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
BSIM Therapeutics, S.A.
US Patent
| Assay Description
In vitro evaluation of inhibitory activity against WT-TTR amyloid fibril formation of AT09 and reference compounds. Reference compounds (thyroxine, t... |
US Patent US10377729 (2019)
BindingDB Entry DOI: 10.7270/Q2WM1GSC |
More data for this
Ligand-Target Pair | |
Transthyretin
(Homo sapiens (Human)) | BDBM408977

(US10377729, Compound AT09-B06)Show InChI InChI=1S/C12H9ClO4/c1-7-9(12(14)15)6-8(17-7)2-3-11-10(13)4-5-16-11/h2-6H,1H3,(H,14,15)/p-1/b3-2+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
BSIM Therapeutics, S.A.
US Patent
| Assay Description
In vitro evaluation of inhibitory activity against WT-TTR amyloid fibril formation of AT09 and reference compounds. Reference compounds (thyroxine, t... |
US Patent US10377729 (2019)
BindingDB Entry DOI: 10.7270/Q2WM1GSC |
More data for this
Ligand-Target Pair | |
Transthyretin
(Homo sapiens (Human)) | BDBM50197883
(CHEBI:78538 | FX-1006 | Tafamidis | US10377729, Co...)Show InChI InChI=1S/C14H7Cl2NO3/c15-9-3-8(4-10(16)6-9)13-17-11-2-1-7(14(18)19)5-12(11)20-13/h1-6H,(H,18,19) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
US Patent
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
BSIM Therapeutics, S.A.
US Patent
| Assay Description
In vitro evaluation of inhibitory activity against WT-TTR amyloid fibril formation of AT09 and reference compounds. Reference compounds (thyroxine, t... |
US Patent US10377729 (2019)
BindingDB Entry DOI: 10.7270/Q2WM1GSC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Homo sapiens (Human)) | BDBM14361

((R,S)-Rolipram | 4-(3-cyclopentyloxy-4-methoxy-phe...)Show InChI InChI=1S/C16H21NO3/c1-19-14-7-6-11(12-9-16(18)17-10-12)8-15(14)20-13-4-2-3-5-13/h6-8,12-13H,2-5,9-10H2,1H3,(H,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4C |
J Med Chem 55: 7525-45 (2012)
Article DOI: 10.1021/jm300514y
BindingDB Entry DOI: 10.7270/Q2CC11TC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Homo sapiens (Human)) | BDBM50396785

(CHEMBL2172707)Show InChI InChI=1S/C8H5ClN2/c9-8-6-3-1-2-4-7(6)10-5-11-8/h1-5H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4C |
J Med Chem 55: 7525-45 (2012)
Article DOI: 10.1021/jm300514y
BindingDB Entry DOI: 10.7270/Q2CC11TC |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50340790
((E)-N-(4-nitrobenzylidene)-2-naphthohydrazide | CH...)Show SMILES [O-][N+](=O)c1ccc(\C=N\NC(=O)c2ccc3ccccc3c2)cc1 Show InChI InChI=1S/C18H13N3O3/c22-18(16-8-7-14-3-1-2-4-15(14)11-16)20-19-12-13-5-9-17(10-6-13)21(23)24/h1-12H,(H,20,22)/b19-12+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IKK-beta expressed in insect Sf21 cells using phospho-Ulight-IkappaB-alpha as substrate |
Eur J Med Chem 46: 1245-53 (2011)
Article DOI: 10.1016/j.ejmech.2011.01.045
BindingDB Entry DOI: 10.7270/Q27M08ZV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data