

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50462179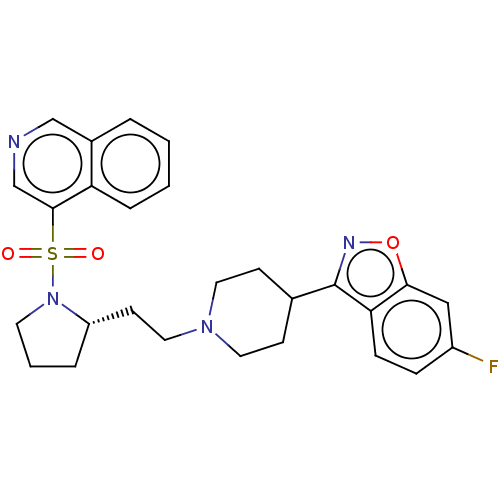 (CHEMBL4245263) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human D2LR expressed in HEK293 cell membranes after 1 hr at 37 degC by microbeta counting method | Eur J Med Chem 145: 790-804 (2018) Article DOI: 10.1016/j.ejmech.2018.01.002 BindingDB Entry DOI: 10.7270/Q27947BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50462156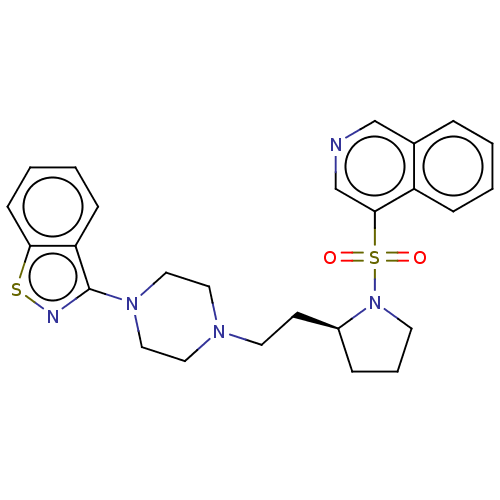 (CHEMBL4246655) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human D2LR expressed in HEK293 cell membranes after 1 hr at 37 degC by microbeta counting method | Eur J Med Chem 145: 790-804 (2018) Article DOI: 10.1016/j.ejmech.2018.01.002 BindingDB Entry DOI: 10.7270/Q27947BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM29563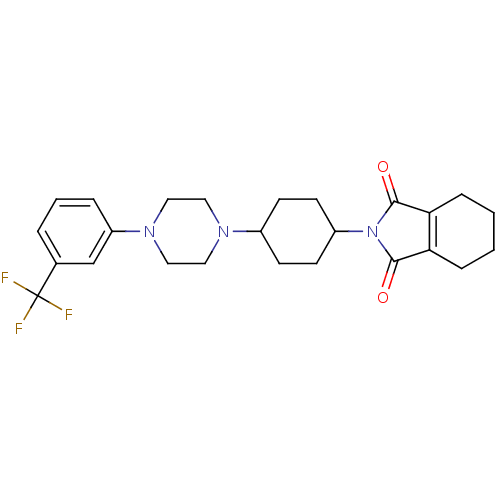 (1-(m-trifluorophenyl)piperazine, 10) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.300 | -56.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Polish Academy of Science | Assay Description Competition experiments were performed in the presence radioligand with membrane protein and test compounds. After incubation, the reaction stopped b... | Bioorg Med Chem 15: 7116-25 (2007) Article DOI: 10.1016/j.bmc.2007.07.029 BindingDB Entry DOI: 10.7270/Q2G15Z6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50462155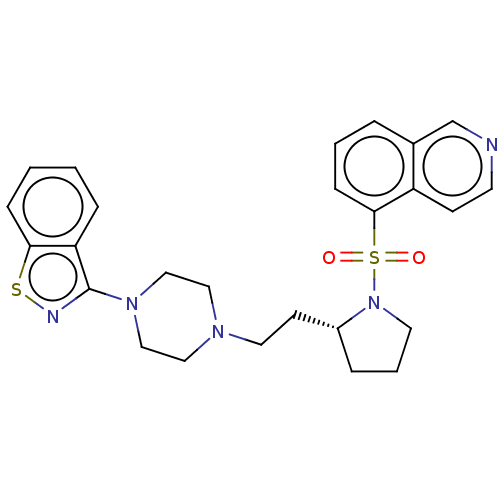 (CHEMBL4239091) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human D2LR expressed in HEK293 cell membranes after 1 hr at 37 degC by microbeta counting method | Eur J Med Chem 145: 790-804 (2018) Article DOI: 10.1016/j.ejmech.2018.01.002 BindingDB Entry DOI: 10.7270/Q27947BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50462179 (CHEMBL4245263) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-5-CT from human 5-HT7R expressed in HEK293 cell membranes after 1 hr at 37 degC by microbeta counting method | Eur J Med Chem 145: 790-804 (2018) Article DOI: 10.1016/j.ejmech.2018.01.002 BindingDB Entry DOI: 10.7270/Q27947BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50001885 ((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-5-CT from human 5-HT7R expressed in HEK293 cell membranes after 1 hr at 37 degC by microbeta counting method | Eur J Med Chem 145: 790-804 (2018) Article DOI: 10.1016/j.ejmech.2018.01.002 BindingDB Entry DOI: 10.7270/Q27947BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50462148 (CHEMBL4242392) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human D2LR expressed in HEK293 cell membranes after 1 hr at 37 degC by microbeta counting method | Eur J Med Chem 145: 790-804 (2018) Article DOI: 10.1016/j.ejmech.2018.01.002 BindingDB Entry DOI: 10.7270/Q27947BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50001885 ((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-ketanserin from human 5-HT2AR expressed in CHO-K1 cell membranes after 1.5 hrs by microbeta counting method | Eur J Med Chem 145: 790-804 (2018) Article DOI: 10.1016/j.ejmech.2018.01.002 BindingDB Entry DOI: 10.7270/Q27947BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50001885 ((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human D2LR expressed in HEK293 cell membranes after 1 hr at 37 degC by microbeta counting method | Eur J Med Chem 145: 790-804 (2018) Article DOI: 10.1016/j.ejmech.2018.01.002 BindingDB Entry DOI: 10.7270/Q27947BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50462142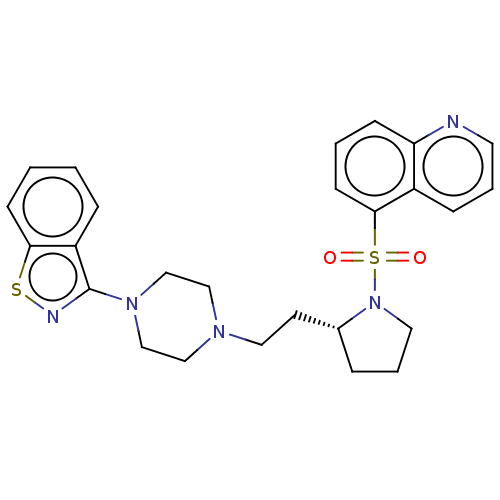 (CHEMBL4242858) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human D2LR expressed in HEK293 cell membranes after 1 hr at 37 degC by microbeta counting method | Eur J Med Chem 145: 790-804 (2018) Article DOI: 10.1016/j.ejmech.2018.01.002 BindingDB Entry DOI: 10.7270/Q27947BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50419052 (SB-399885) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6 receptor expressed in HEK293 cell membranes measured after 1 hr by microbeta counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00224 BindingDB Entry DOI: 10.7270/Q2X3528B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50048803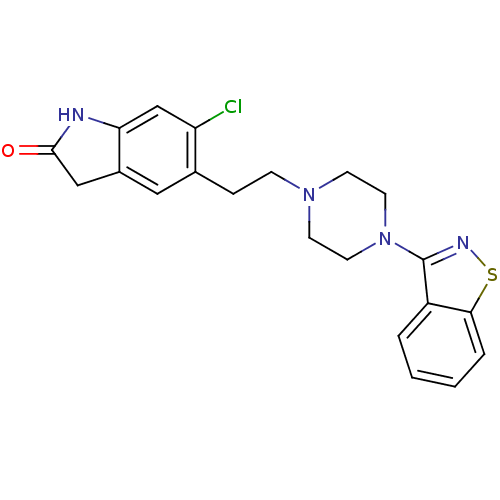 (5-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human D2LR expressed in HEK293 cell membranes after 1 hr at 37 degC by microbeta counting method | Eur J Med Chem 145: 790-804 (2018) Article DOI: 10.1016/j.ejmech.2018.01.002 BindingDB Entry DOI: 10.7270/Q27947BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50130293 (7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human D2LR expressed in HEK293 cell membranes after 1 hr at 37 degC by microbeta counting method | Eur J Med Chem 145: 790-804 (2018) Article DOI: 10.1016/j.ejmech.2018.01.002 BindingDB Entry DOI: 10.7270/Q27947BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50048803 (5-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.851 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]-methylspiperone from human D2 long receptor expressed in HEK293 cells measured after 1 hr by liquid scintillation counter method | Eur J Med Chem 179: 1-15 (2019) Article DOI: 10.1016/j.ejmech.2019.06.001 BindingDB Entry DOI: 10.7270/Q2S185TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50048803 (5-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]-methylspiperone from human D2 long receptor expressed in HEK293 cells measured after 1 hr by liquid scintillation counter method | Eur J Med Chem 179: 1-15 (2019) Article DOI: 10.1016/j.ejmech.2019.06.001 BindingDB Entry DOI: 10.7270/Q2S185TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50462152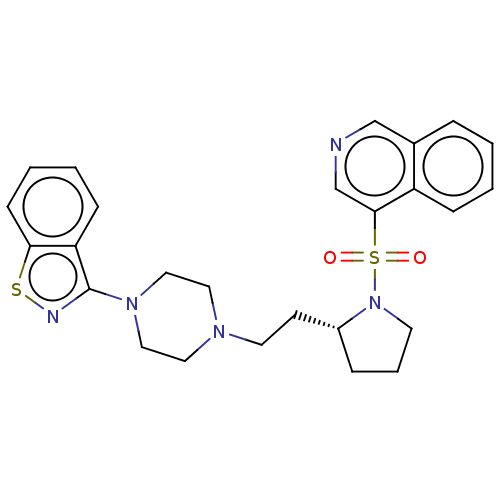 (CHEMBL4238679) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human D2LR expressed in HEK293 cell membranes after 1 hr at 37 degC by microbeta counting method | Eur J Med Chem 145: 790-804 (2018) Article DOI: 10.1016/j.ejmech.2018.01.002 BindingDB Entry DOI: 10.7270/Q27947BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50579346 (CHEMBL4867565) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to 5HT3 receptor (unknown origin) | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00224 BindingDB Entry DOI: 10.7270/Q2X3528B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50462168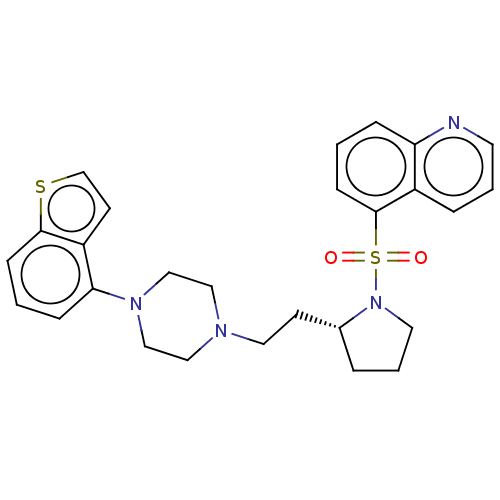 (CHEMBL4243848) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-5-CT from human 5-HT7R expressed in HEK293 cell membranes after 1 hr at 37 degC by microbeta counting method | Eur J Med Chem 145: 790-804 (2018) Article DOI: 10.1016/j.ejmech.2018.01.002 BindingDB Entry DOI: 10.7270/Q27947BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50462142 (CHEMBL4242858) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-5-CT from human 5-HT7R expressed in HEK293 cell membranes after 1 hr at 37 degC by microbeta counting method | Eur J Med Chem 145: 790-804 (2018) Article DOI: 10.1016/j.ejmech.2018.01.002 BindingDB Entry DOI: 10.7270/Q27947BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50462138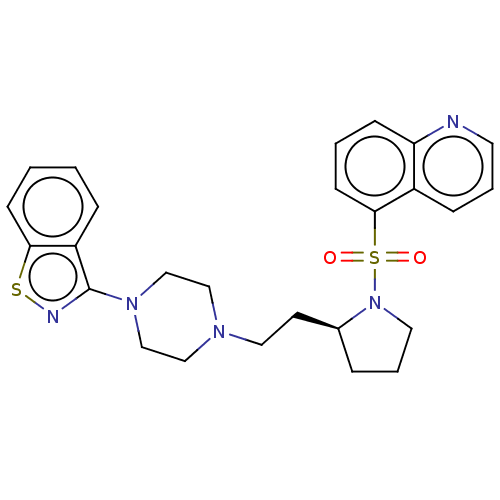 (CHEMBL4246202) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human D2LR expressed in HEK293 cell membranes after 1 hr at 37 degC by microbeta counting method | Eur J Med Chem 145: 790-804 (2018) Article DOI: 10.1016/j.ejmech.2018.01.002 BindingDB Entry DOI: 10.7270/Q27947BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50462155 (CHEMBL4239091) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-5-CT from human 5-HT7R expressed in HEK293 cell membranes after 1 hr at 37 degC by microbeta counting method | Eur J Med Chem 145: 790-804 (2018) Article DOI: 10.1016/j.ejmech.2018.01.002 BindingDB Entry DOI: 10.7270/Q27947BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM29567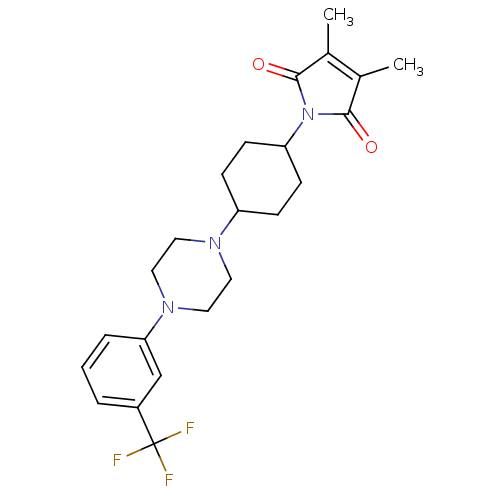 (1-(m-trifluorophenyl)piperazine, 14) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.30 | -52.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Polish Academy of Science | Assay Description Competition experiments were performed in the presence radioligand with membrane protein and test compounds. After incubation, the reaction stopped b... | Bioorg Med Chem 15: 7116-25 (2007) Article DOI: 10.1016/j.bmc.2007.07.029 BindingDB Entry DOI: 10.7270/Q2G15Z6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50318633 (3-benzenesulfonyl-8-piperazin-1-ylquinoline | CHEM...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6 receptor expressed in HEK293 cell membranes measured after 1 hr by microbeta counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00224 BindingDB Entry DOI: 10.7270/Q2X3528B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM29565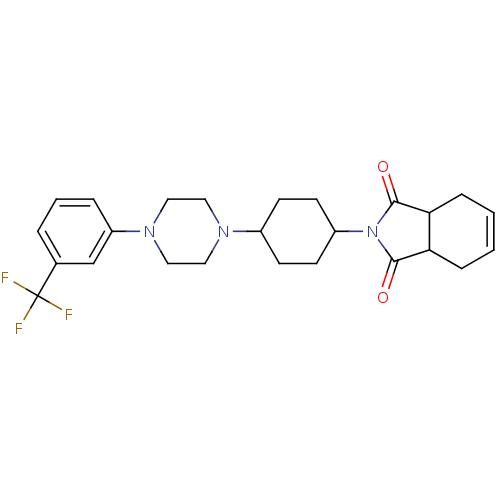 (1-(m-trifluorophenyl)piperazine, 12) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.5 | -52.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Polish Academy of Science | Assay Description Competition experiments were performed in the presence radioligand with membrane protein and test compounds. After incubation, the reaction stopped b... | Bioorg Med Chem 15: 7116-25 (2007) Article DOI: 10.1016/j.bmc.2007.07.029 BindingDB Entry DOI: 10.7270/Q2G15Z6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50462169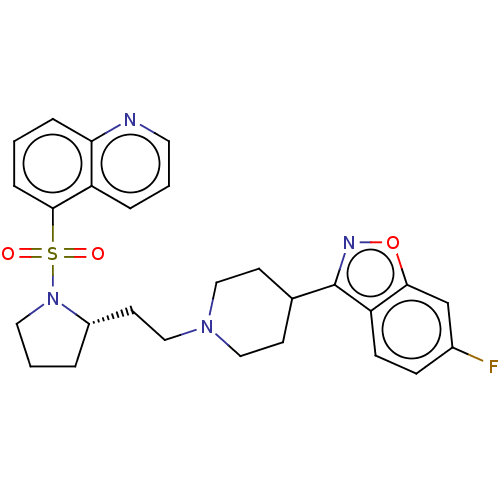 (CHEMBL4241050) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human D2LR expressed in HEK293 cell membranes after 1 hr at 37 degC by microbeta counting method | Eur J Med Chem 145: 790-804 (2018) Article DOI: 10.1016/j.ejmech.2018.01.002 BindingDB Entry DOI: 10.7270/Q27947BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50048803 (5-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)et...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-ketanserin from human 5-HT2AR expressed in CHO-K1 cell membranes after 1.5 hrs by microbeta counting method | Eur J Med Chem 145: 790-804 (2018) Article DOI: 10.1016/j.ejmech.2018.01.002 BindingDB Entry DOI: 10.7270/Q27947BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50513415 (CHEMBL4435010) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells measured after 1 hr by liquid scintillation counter method | Eur J Med Chem 179: 1-15 (2019) Article DOI: 10.1016/j.ejmech.2019.06.001 BindingDB Entry DOI: 10.7270/Q2S185TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50579339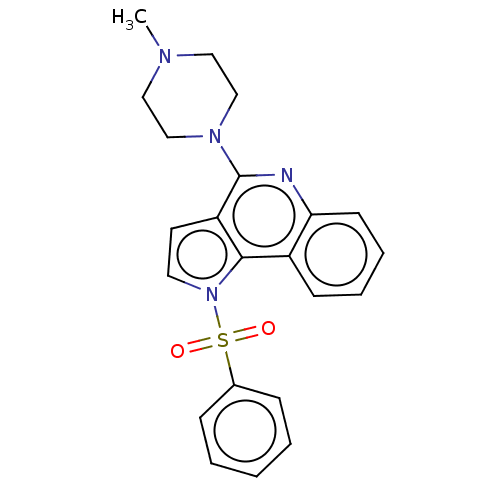 (CHEMBL4865309) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6 receptor expressed in HEK293 cell membranes measured after 1 hr by microbeta counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00224 BindingDB Entry DOI: 10.7270/Q2X3528B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50579341 (CHEMBL4860809) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6 receptor expressed in HEK293 cell membranes measured after 1 hr by microbeta counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00224 BindingDB Entry DOI: 10.7270/Q2X3528B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50579342 (CHEMBL4872586) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6 receptor expressed in HEK293 cell membranes measured after 1 hr by microbeta counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00224 BindingDB Entry DOI: 10.7270/Q2X3528B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50048803 (5-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)et...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1AR expressed in HEK293 cell membranes after 1 hr by microbeta counting method | Eur J Med Chem 145: 790-804 (2018) Article DOI: 10.1016/j.ejmech.2018.01.002 BindingDB Entry DOI: 10.7270/Q27947BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human D2LR expressed in HEK293 cell membranes after 1 hr at 37 degC by microbeta counting method | Eur J Med Chem 145: 790-804 (2018) Article DOI: 10.1016/j.ejmech.2018.01.002 BindingDB Entry DOI: 10.7270/Q27947BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50462177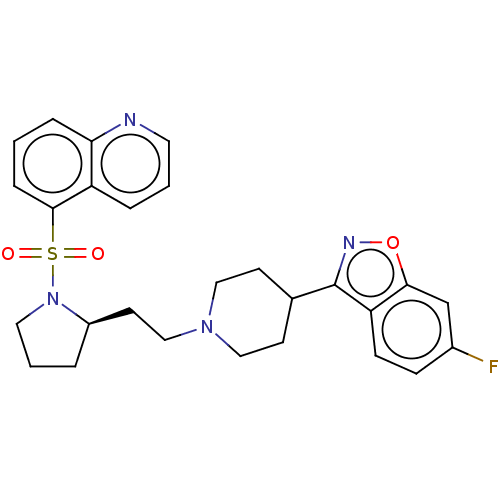 (CHEMBL4250475) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human D2LR expressed in HEK293 cell membranes after 1 hr at 37 degC by microbeta counting method | Eur J Med Chem 145: 790-804 (2018) Article DOI: 10.1016/j.ejmech.2018.01.002 BindingDB Entry DOI: 10.7270/Q27947BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM29566 (1-(m-trifluorophenyl)piperazine, 13) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.20 | -51.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Polish Academy of Science | Assay Description Competition experiments were performed in the presence radioligand with membrane protein and test compounds. After incubation, the reaction stopped b... | Bioorg Med Chem 15: 7116-25 (2007) Article DOI: 10.1016/j.bmc.2007.07.029 BindingDB Entry DOI: 10.7270/Q2G15Z6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM29561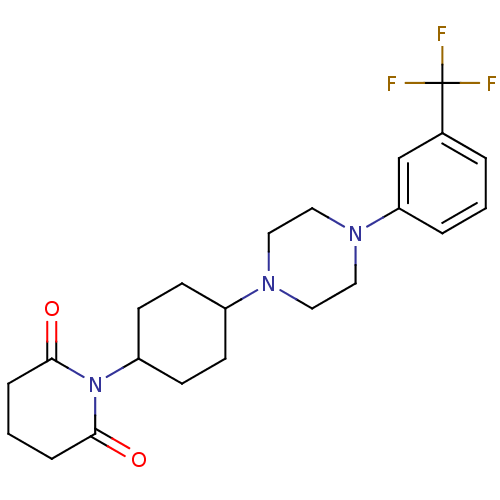 (1-(m-trifluorophenyl)piperazine, 8) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.60 | -51.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Polish Academy of Science | Assay Description Competition experiments were performed in the presence radioligand with membrane protein and test compounds. After incubation, the reaction stopped b... | Bioorg Med Chem 15: 7116-25 (2007) Article DOI: 10.1016/j.bmc.2007.07.029 BindingDB Entry DOI: 10.7270/Q2G15Z6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50146089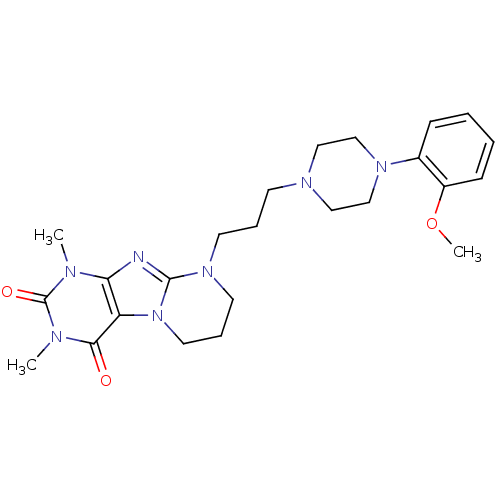 (1,3-dimethyl-9-{3-[4-(2'-methoxyphenyl)-piperazin-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5HT1A receptor in rat cerebral cortex after 15 mins by liquid scintillation counting | Eur J Med Chem 44: 4288-96 (2009) Article DOI: 10.1016/j.ejmech.2009.07.014 BindingDB Entry DOI: 10.7270/Q2C53KWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50274767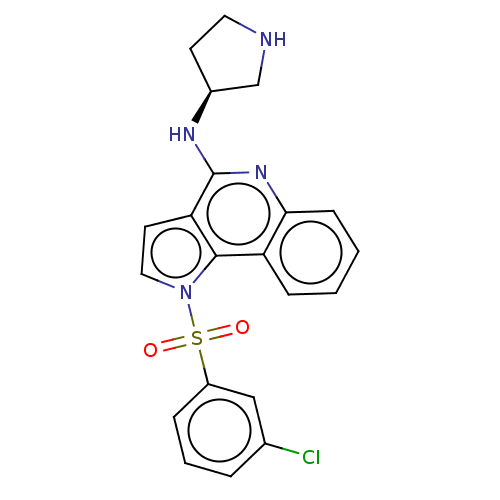 (CHEMBL4125735) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6 receptor expressed in HEK293 cell membranes measured after 1 hr by microbeta counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00224 BindingDB Entry DOI: 10.7270/Q2X3528B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50206353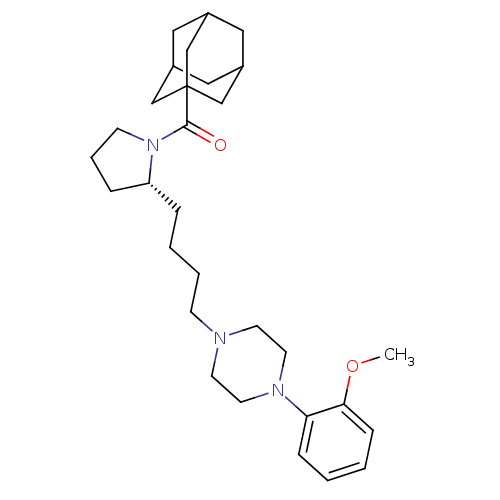 (1-(adamantylcarbonyl)-N-{4-[4-(2-methoxyphenyl)pip...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Montpellier I et II Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from serotonin 5HT1A receptor in rat brain hippocampus | Bioorg Med Chem 15: 2907-19 (2007) Article DOI: 10.1016/j.bmc.2007.02.018 BindingDB Entry DOI: 10.7270/Q20G3JTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50513432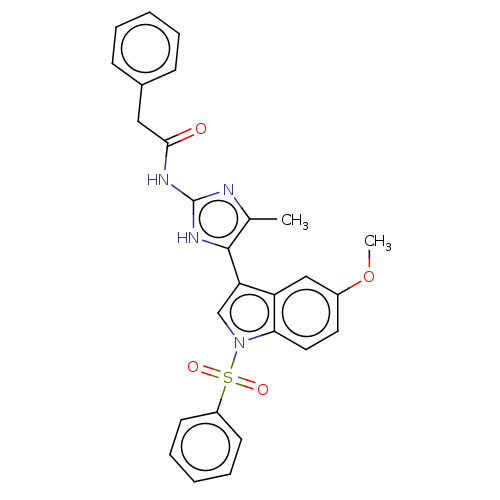 (CHEMBL4586990) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells measured after 1 hr by liquid scintillation counter method | Eur J Med Chem 179: 1-15 (2019) Article DOI: 10.1016/j.ejmech.2019.06.001 BindingDB Entry DOI: 10.7270/Q2S185TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50579346 (CHEMBL4867565) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6 receptor expressed in HEK293 cell membranes measured after 1 hr by microbeta counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00224 BindingDB Entry DOI: 10.7270/Q2X3528B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50579347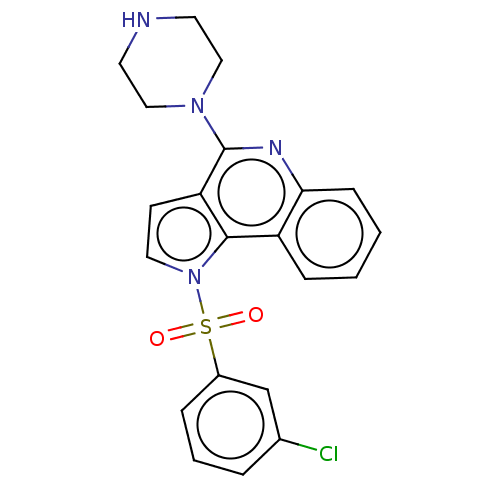 (CHEMBL4846153) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6 receptor expressed in HEK293 cell membranes measured after 1 hr by microbeta counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00224 BindingDB Entry DOI: 10.7270/Q2X3528B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50579348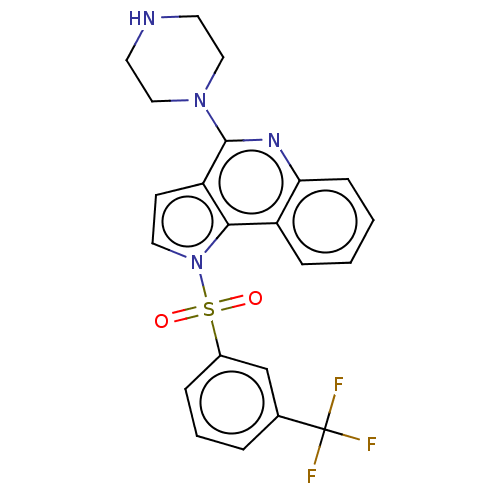 (CHEMBL4862354) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6 receptor expressed in HEK293 cell membranes measured after 1 hr by microbeta counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00224 BindingDB Entry DOI: 10.7270/Q2X3528B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50579349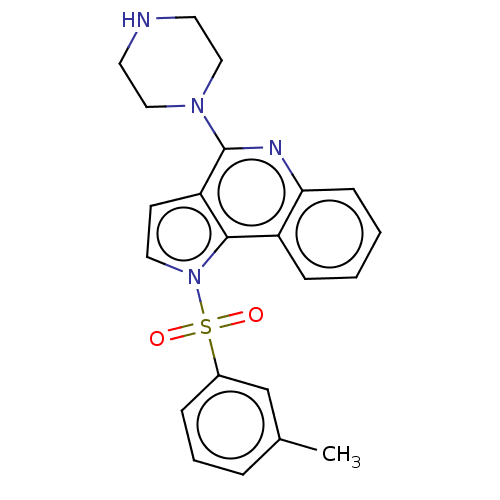 (CHEMBL4870374) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6 receptor expressed in HEK293 cell membranes measured after 1 hr by microbeta counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00224 BindingDB Entry DOI: 10.7270/Q2X3528B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50462137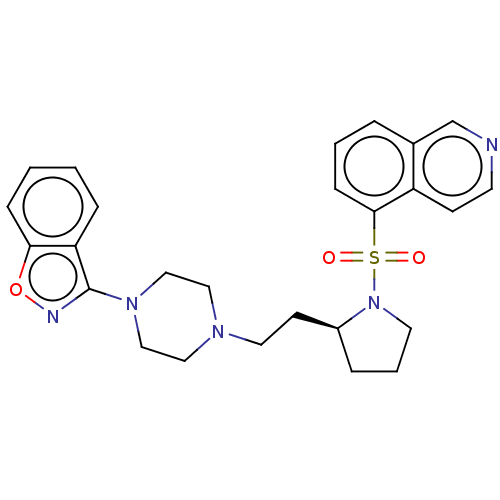 (CHEMBL4243579) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human D2LR expressed in HEK293 cell membranes after 1 hr at 37 degC by microbeta counting method | Eur J Med Chem 145: 790-804 (2018) Article DOI: 10.1016/j.ejmech.2018.01.002 BindingDB Entry DOI: 10.7270/Q27947BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50462151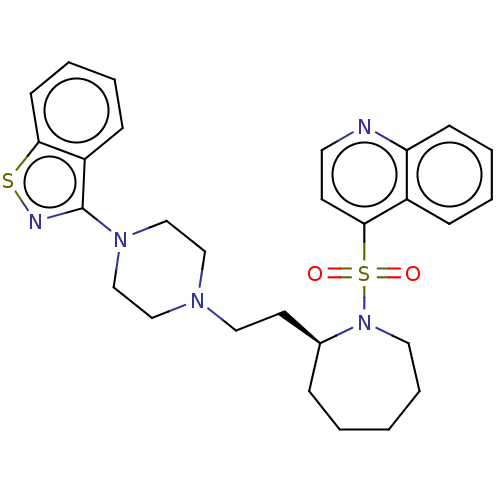 (CHEMBL4250274) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human D2LR expressed in HEK293 cell membranes after 1 hr at 37 degC by microbeta counting method | Eur J Med Chem 145: 790-804 (2018) Article DOI: 10.1016/j.ejmech.2018.01.002 BindingDB Entry DOI: 10.7270/Q27947BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50462178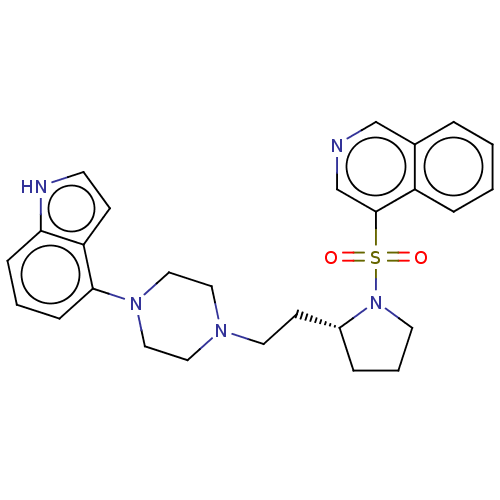 (CHEMBL4245997) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human D2LR expressed in HEK293 cell membranes after 1 hr at 37 degC by microbeta counting method | Eur J Med Chem 145: 790-804 (2018) Article DOI: 10.1016/j.ejmech.2018.01.002 BindingDB Entry DOI: 10.7270/Q27947BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50462181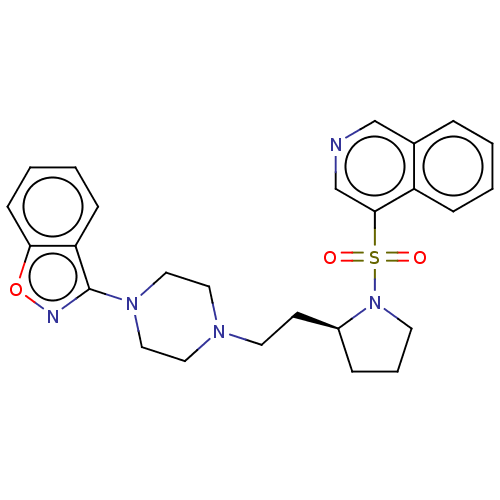 (CHEMBL4249640) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human D2LR expressed in HEK293 cell membranes after 1 hr at 37 degC by microbeta counting method | Eur J Med Chem 145: 790-804 (2018) Article DOI: 10.1016/j.ejmech.2018.01.002 BindingDB Entry DOI: 10.7270/Q27947BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50462173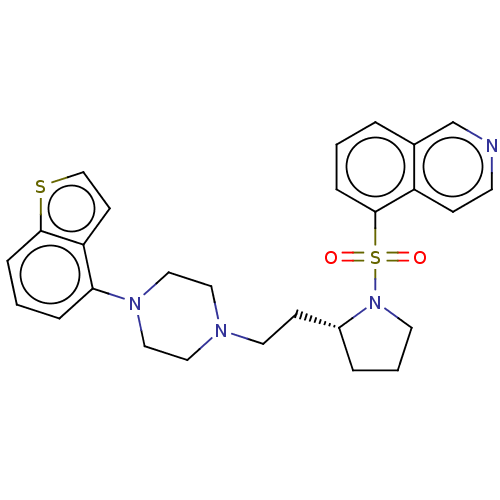 (CHEMBL4238479) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-5-CT from human 5-HT7R expressed in HEK293 cell membranes after 1 hr at 37 degC by microbeta counting method | Eur J Med Chem 145: 790-804 (2018) Article DOI: 10.1016/j.ejmech.2018.01.002 BindingDB Entry DOI: 10.7270/Q27947BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50462152 (CHEMBL4238679) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-5-CT from human 5-HT7R expressed in HEK293 cell membranes after 1 hr at 37 degC by microbeta counting method | Eur J Med Chem 145: 790-804 (2018) Article DOI: 10.1016/j.ejmech.2018.01.002 BindingDB Entry DOI: 10.7270/Q27947BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM29560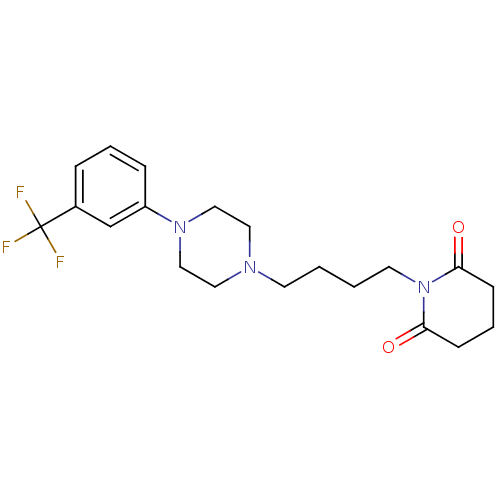 (1-(m-trifluorophenyl)piperazine, 7) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | -50.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Polish Academy of Science | Assay Description Competition experiments were performed in the presence radioligand with membrane protein and test compounds. After incubation, the reaction stopped b... | Bioorg Med Chem 15: 7116-25 (2007) Article DOI: 10.1016/j.bmc.2007.07.029 BindingDB Entry DOI: 10.7270/Q2G15Z6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 732 total ) | Next | Last >> |