

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50282499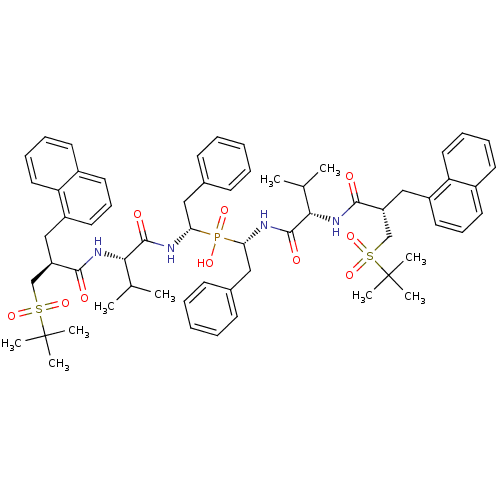 (Bis-((R)-1-{(S)-3-methyl-2-[(S)-3-(2-methyl-propan...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity if the compound was determined against HIV protease | Bioorg Med Chem Lett 4: 1191-1194 (1994) Article DOI: 10.1016/S0960-894X(01)80327-9 BindingDB Entry DOI: 10.7270/Q28G8KM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50282499 (Bis-((R)-1-{(S)-3-methyl-2-[(S)-3-(2-methyl-propan...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity if the compound was determined against HIV protease | Bioorg Med Chem Lett 4: 1191-1194 (1994) Article DOI: 10.1016/S0960-894X(01)80327-9 BindingDB Entry DOI: 10.7270/Q28G8KM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50282495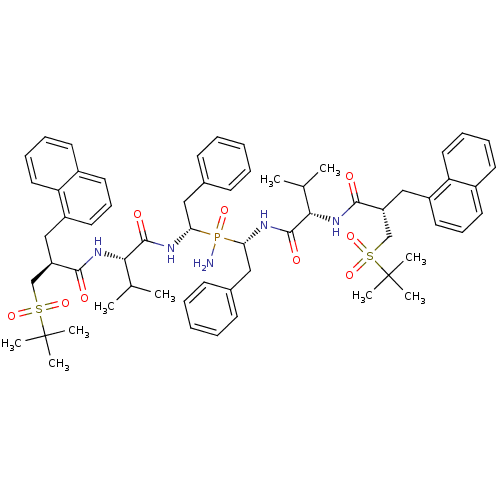 (Bis-((R)-1-{(S)-3-methyl-2-[(S)-3-(2-methyl-propan...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity if the compound was determined against HIV protease | Bioorg Med Chem Lett 4: 1191-1194 (1994) Article DOI: 10.1016/S0960-894X(01)80327-9 BindingDB Entry DOI: 10.7270/Q28G8KM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50282495 (Bis-((R)-1-{(S)-3-methyl-2-[(S)-3-(2-methyl-propan...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity if the compound was determined against HIV protease | Bioorg Med Chem Lett 4: 1191-1194 (1994) Article DOI: 10.1016/S0960-894X(01)80327-9 BindingDB Entry DOI: 10.7270/Q28G8KM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50282500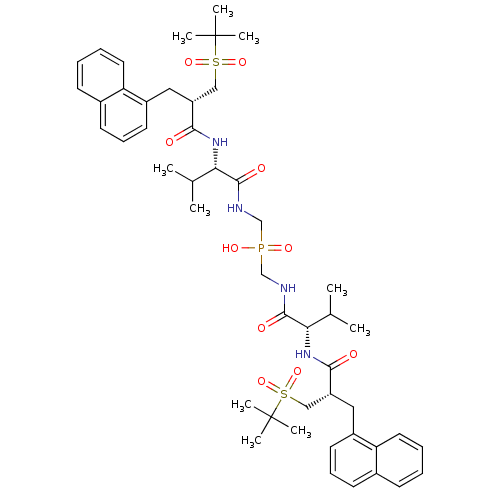 (({(S)-3-Methyl-2-[2-((S)-2-methyl-propane-2-sulfon...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity if the compound was determined against HIV protease | Bioorg Med Chem Lett 4: 1191-1194 (1994) Article DOI: 10.1016/S0960-894X(01)80327-9 BindingDB Entry DOI: 10.7270/Q28G8KM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50282496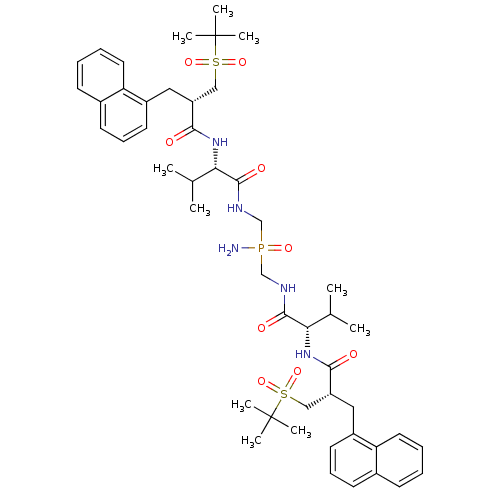 (Bis-({(S)-3-methyl-2-[(S)-3-(2-methyl-propane-2-su...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity if the compound was determined against HIV protease | Bioorg Med Chem Lett 4: 1191-1194 (1994) Article DOI: 10.1016/S0960-894X(01)80327-9 BindingDB Entry DOI: 10.7270/Q28G8KM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50282498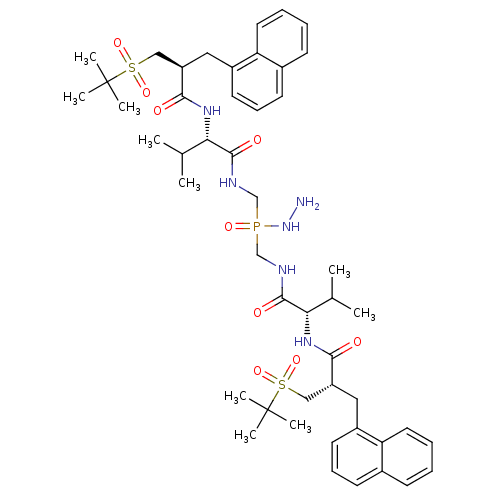 (Bis-({(S)-3-methyl-2-[(S)-3-(2-methyl-propane-2-su...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity if the compound was determined against HIV protease | Bioorg Med Chem Lett 4: 1191-1194 (1994) Article DOI: 10.1016/S0960-894X(01)80327-9 BindingDB Entry DOI: 10.7270/Q28G8KM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50282497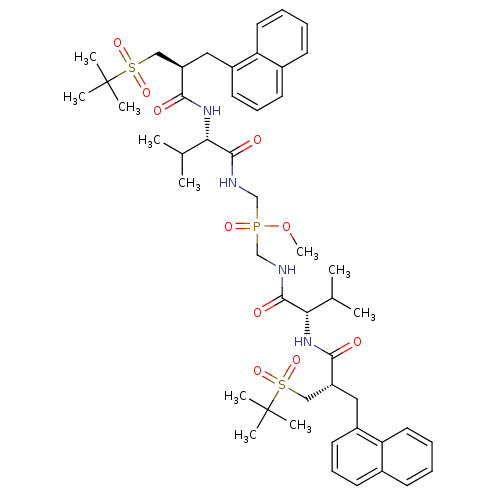 (Bis-({(S)-3-methyl-2-[(S)-3-(2-methyl-propane-2-su...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity if the compound was determined against HIV protease | Bioorg Med Chem Lett 4: 1191-1194 (1994) Article DOI: 10.1016/S0960-894X(01)80327-9 BindingDB Entry DOI: 10.7270/Q28G8KM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||