 Found 107 hits with Last Name = 'rudra' and Initial = 'a'
Found 107 hits with Last Name = 'rudra' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50557805
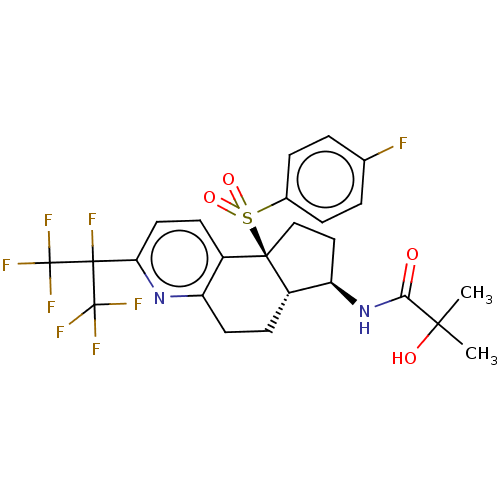
(CHEMBL4750756)Show SMILES [H][C@@]12CCc3nc(ccc3[C@@]1(CC[C@H]2NC(=O)C(C)(C)O)S(=O)(=O)c1ccc(F)cc1)C(F)(C(F)(F)F)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00112
BindingDB Entry DOI: 10.7270/Q21R6V6T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50557811
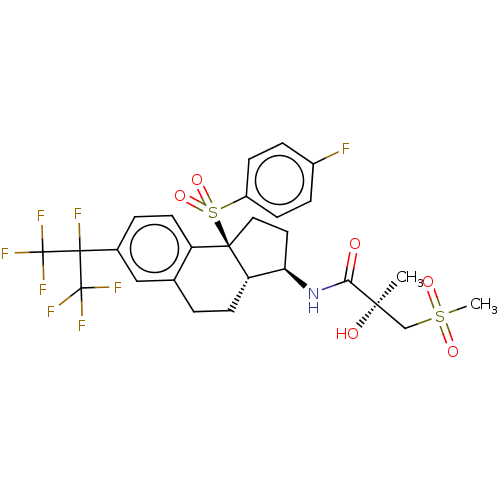
(CHEMBL4779994)Show SMILES [H][C@@]12CCc3cc(ccc3[C@@]1(CC[C@H]2NC(=O)[C@](C)(O)CS(C)(=O)=O)S(=O)(=O)c1ccc(F)cc1)C(F)(C(F)(F)F)C(F)(F)F |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00112
BindingDB Entry DOI: 10.7270/Q21R6V6T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM414262
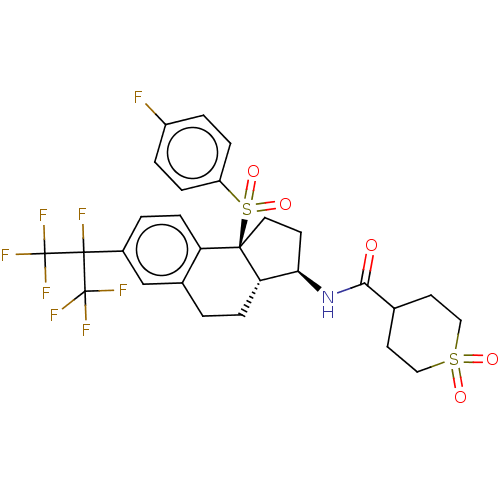
(US10435369, Example 149)Show SMILES Fc1ccc(cc1)S(=O)(=O)[C@@]12CC[C@@H](NC(=O)C3CCS(=O)(=O)CC3)[C@@H]1CCc1cc(ccc21)C(F)(C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C28H27F8NO5S2/c29-19-3-5-20(6-4-19)44(41,42)25-12-9-23(37-24(38)16-10-13-43(39,40)14-11-16)22(25)7-1-17-15-18(2-8-21(17)25)26(30,27(31,32)33)28(34,35)36/h2-6,8,15-16,22-23H,1,7,9-14H2,(H,37,38)/t22-,23+,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00112
BindingDB Entry DOI: 10.7270/Q21R6V6T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM414262

(US10435369, Example 149)Show SMILES Fc1ccc(cc1)S(=O)(=O)[C@@]12CC[C@@H](NC(=O)C3CCS(=O)(=O)CC3)[C@@H]1CCc1cc(ccc21)C(F)(C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C28H27F8NO5S2/c29-19-3-5-20(6-4-19)44(41,42)25-12-9-23(37-24(38)16-10-13-43(39,40)14-11-16)22(25)7-1-17-15-18(2-8-21(17)25)26(30,27(31,32)33)28(34,35)36/h2-6,8,15-16,22-23H,1,7,9-14H2,(H,37,38)/t22-,23+,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00112
BindingDB Entry DOI: 10.7270/Q21R6V6T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50557806
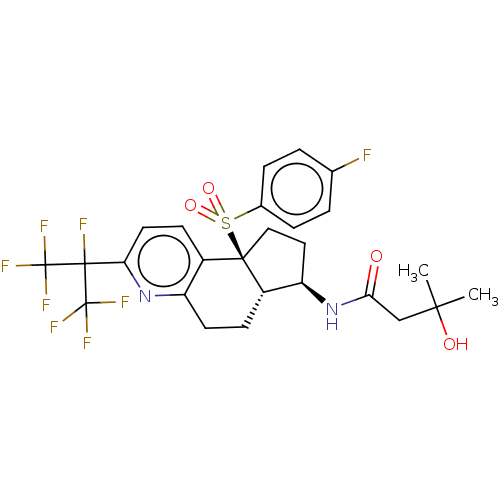
(CHEMBL4800380)Show SMILES [H][C@@]12CCc3nc(ccc3[C@@]1(CC[C@H]2NC(=O)CC(C)(C)O)S(=O)(=O)c1ccc(F)cc1)C(F)(C(F)(F)F)C(F)(F)F |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00112
BindingDB Entry DOI: 10.7270/Q21R6V6T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM414262

(US10435369, Example 149)Show SMILES Fc1ccc(cc1)S(=O)(=O)[C@@]12CC[C@@H](NC(=O)C3CCS(=O)(=O)CC3)[C@@H]1CCc1cc(ccc21)C(F)(C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C28H27F8NO5S2/c29-19-3-5-20(6-4-19)44(41,42)25-12-9-23(37-24(38)16-10-13-43(39,40)14-11-16)22(25)7-1-17-15-18(2-8-21(17)25)26(30,27(31,32)33)28(34,35)36/h2-6,8,15-16,22-23H,1,7,9-14H2,(H,37,38)/t22-,23+,25+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00112
BindingDB Entry DOI: 10.7270/Q21R6V6T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50557809

(CHEMBL4760466)Show SMILES [H][C@@]12CCc3nc(ccc3[C@@]1(CC[C@H]2NC(=O)[C@](C)(O)CS(C)(=O)=O)S(=O)(=O)c1ccc(F)cc1)C(F)(C(F)(F)F)C(F)(F)F |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00112
BindingDB Entry DOI: 10.7270/Q21R6V6T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM414277
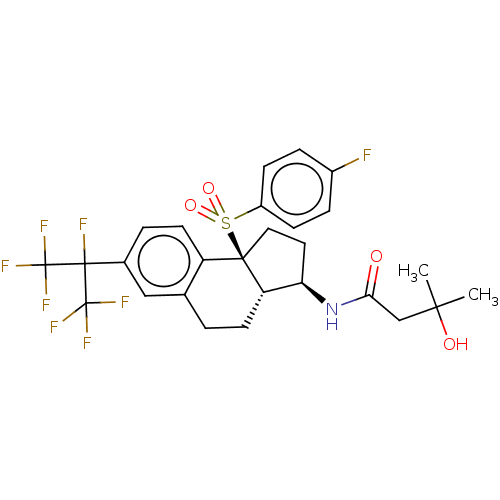
(US10435369, Example 164)Show SMILES CC(C)(O)CC(=O)N[C@@H]1CC[C@@]2([C@H]1CCc1cc(ccc21)C(F)(C(F)(F)F)C(F)(F)F)S(=O)(=O)c1ccc(F)cc1 |r| Show InChI InChI=1S/C27H27F8NO4S/c1-23(2,38)14-22(37)36-21-11-12-24(41(39,40)18-7-5-17(28)6-8-18)19-10-4-16(13-15(19)3-9-20(21)24)25(29,26(30,31)32)27(33,34)35/h4-8,10,13,20-21,38H,3,9,11-12,14H2,1-2H3,(H,36,37)/t20-,21+,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00112
BindingDB Entry DOI: 10.7270/Q21R6V6T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50557805

(CHEMBL4750756)Show SMILES [H][C@@]12CCc3nc(ccc3[C@@]1(CC[C@H]2NC(=O)C(C)(C)O)S(=O)(=O)c1ccc(F)cc1)C(F)(C(F)(F)F)C(F)(F)F |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00112
BindingDB Entry DOI: 10.7270/Q21R6V6T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50557806

(CHEMBL4800380)Show SMILES [H][C@@]12CCc3nc(ccc3[C@@]1(CC[C@H]2NC(=O)CC(C)(C)O)S(=O)(=O)c1ccc(F)cc1)C(F)(C(F)(F)F)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00112
BindingDB Entry DOI: 10.7270/Q21R6V6T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50557806

(CHEMBL4800380)Show SMILES [H][C@@]12CCc3nc(ccc3[C@@]1(CC[C@H]2NC(=O)CC(C)(C)O)S(=O)(=O)c1ccc(F)cc1)C(F)(C(F)(F)F)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00112
BindingDB Entry DOI: 10.7270/Q21R6V6T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50557810

(CHEMBL4741364)Show SMILES [H][C@@]12CCc3cc(ccc3[C@@]1(CC[C@H]2NC(=O)[C@@](C)(O)CS(C)(=O)=O)S(=O)(=O)c1ccc(F)cc1)C(F)(C(F)(F)F)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00112
BindingDB Entry DOI: 10.7270/Q21R6V6T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM382195
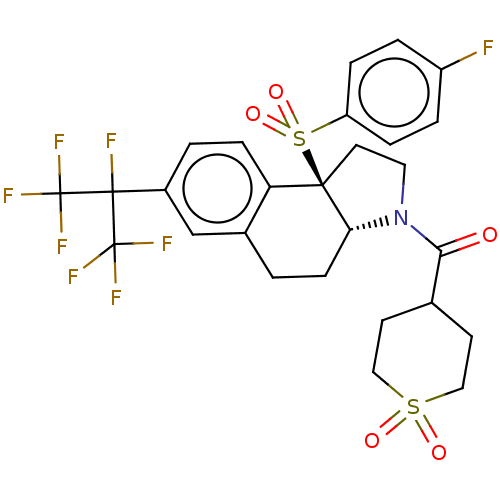
(US10273259, Example 11 | US10711020, Example 11)Show SMILES Fc1ccc(cc1)S(=O)(=O)[C@@]12CCN([C@@H]1CCc1cc(ccc21)C(F)(C(F)(F)F)C(F)(F)F)C(=O)C1CCS(=O)(=O)CC1 |r| Show InChI InChI=1S/C27H25F8NO5S2/c28-19-3-5-20(6-4-19)43(40,41)24-11-12-36(23(37)16-9-13-42(38,39)14-10-16)22(24)8-1-17-15-18(2-7-21(17)24)25(29,26(30,31)32)27(33,34)35/h2-7,15-16,22H,1,8-14H2/t22-,24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00112
BindingDB Entry DOI: 10.7270/Q21R6V6T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50557807
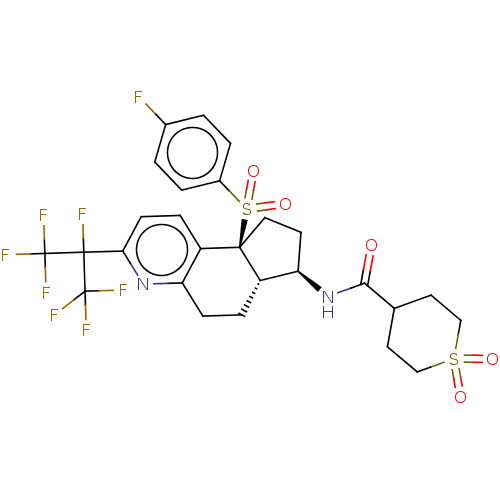
(CHEMBL4744956)Show SMILES [H][C@@]12CCc3nc(ccc3[C@@]1(CC[C@H]2NC(=O)C1CCS(=O)(=O)CC1)S(=O)(=O)c1ccc(F)cc1)C(F)(C(F)(F)F)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00112
BindingDB Entry DOI: 10.7270/Q21R6V6T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM383152
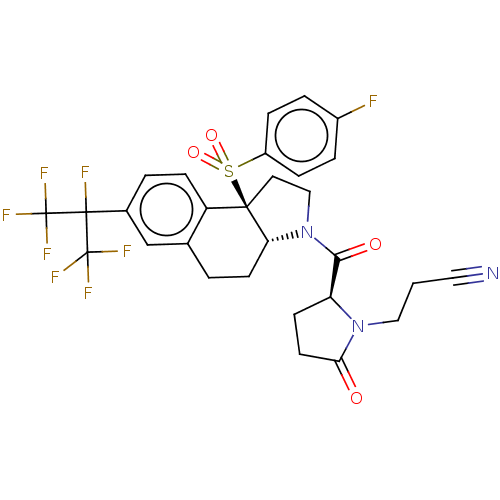
(US10273259, Example 863 | US10711020, Example 863)Show SMILES Fc1ccc(cc1)S(=O)(=O)[C@@]12CCN([C@@H]1CCc1cc(ccc21)C(F)(C(F)(F)F)C(F)(F)F)C(=O)[C@@H]1CCC(=O)N1CCC#N |r| Show InChI InChI=1S/C29H25F8N3O4S/c30-19-4-6-20(7-5-19)45(43,44)26-12-15-40(25(42)22-9-11-24(41)39(22)14-1-13-38)23(26)10-2-17-16-18(3-8-21(17)26)27(31,28(32,33)34)29(35,36)37/h3-8,16,22-23H,1-2,9-12,14-15H2/t22-,23+,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00112
BindingDB Entry DOI: 10.7270/Q21R6V6T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM414277

(US10435369, Example 164)Show SMILES CC(C)(O)CC(=O)N[C@@H]1CC[C@@]2([C@H]1CCc1cc(ccc21)C(F)(C(F)(F)F)C(F)(F)F)S(=O)(=O)c1ccc(F)cc1 |r| Show InChI InChI=1S/C27H27F8NO4S/c1-23(2,38)14-22(37)36-21-11-12-24(41(39,40)18-7-5-17(28)6-8-18)19-10-4-16(13-15(19)3-9-20(21)24)25(29,26(30,31)32)27(33,34)35/h4-8,10,13,20-21,38H,3,9,11-12,14H2,1-2H3,(H,36,37)/t20-,21+,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00112
BindingDB Entry DOI: 10.7270/Q21R6V6T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM414277

(US10435369, Example 164)Show SMILES CC(C)(O)CC(=O)N[C@@H]1CC[C@@]2([C@H]1CCc1cc(ccc21)C(F)(C(F)(F)F)C(F)(F)F)S(=O)(=O)c1ccc(F)cc1 |r| Show InChI InChI=1S/C27H27F8NO4S/c1-23(2,38)14-22(37)36-21-11-12-24(41(39,40)18-7-5-17(28)6-8-18)19-10-4-16(13-15(19)3-9-20(21)24)25(29,26(30,31)32)27(33,34)35/h4-8,10,13,20-21,38H,3,9,11-12,14H2,1-2H3,(H,36,37)/t20-,21+,24+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00112
BindingDB Entry DOI: 10.7270/Q21R6V6T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50557802
(CHEMBL4748187)Show SMILES [H][C@@]12CCc3cc(ncc3[C@@]1(CCN2C(=O)[C@@H]1CCC(=O)N1CCC#N)S(=O)(=O)c1ccc(F)cc1)C(F)(C(F)(F)F)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00112
BindingDB Entry DOI: 10.7270/Q21R6V6T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM414277

(US10435369, Example 164)Show SMILES CC(C)(O)CC(=O)N[C@@H]1CC[C@@]2([C@H]1CCc1cc(ccc21)C(F)(C(F)(F)F)C(F)(F)F)S(=O)(=O)c1ccc(F)cc1 |r| Show InChI InChI=1S/C27H27F8NO4S/c1-23(2,38)14-22(37)36-21-11-12-24(41(39,40)18-7-5-17(28)6-8-18)19-10-4-16(13-15(19)3-9-20(21)24)25(29,26(30,31)32)27(33,34)35/h4-8,10,13,20-21,38H,3,9,11-12,14H2,1-2H3,(H,36,37)/t20-,21+,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00112
BindingDB Entry DOI: 10.7270/Q21R6V6T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50557811

(CHEMBL4779994)Show SMILES [H][C@@]12CCc3cc(ccc3[C@@]1(CC[C@H]2NC(=O)[C@](C)(O)CS(C)(=O)=O)S(=O)(=O)c1ccc(F)cc1)C(F)(C(F)(F)F)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00112
BindingDB Entry DOI: 10.7270/Q21R6V6T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM383152

(US10273259, Example 863 | US10711020, Example 863)Show SMILES Fc1ccc(cc1)S(=O)(=O)[C@@]12CCN([C@@H]1CCc1cc(ccc21)C(F)(C(F)(F)F)C(F)(F)F)C(=O)[C@@H]1CCC(=O)N1CCC#N |r| Show InChI InChI=1S/C29H25F8N3O4S/c30-19-4-6-20(7-5-19)45(43,44)26-12-15-40(25(42)22-9-11-24(41)39(22)14-1-13-38)23(26)10-2-17-16-18(3-8-21(17)26)27(31,28(32,33)34)29(35,36)37/h3-8,16,22-23H,1-2,9-12,14-15H2/t22-,23+,26+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00112
BindingDB Entry DOI: 10.7270/Q21R6V6T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50557810

(CHEMBL4741364)Show SMILES [H][C@@]12CCc3cc(ccc3[C@@]1(CC[C@H]2NC(=O)[C@@](C)(O)CS(C)(=O)=O)S(=O)(=O)c1ccc(F)cc1)C(F)(C(F)(F)F)C(F)(F)F |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00112
BindingDB Entry DOI: 10.7270/Q21R6V6T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM382195

(US10273259, Example 11 | US10711020, Example 11)Show SMILES Fc1ccc(cc1)S(=O)(=O)[C@@]12CCN([C@@H]1CCc1cc(ccc21)C(F)(C(F)(F)F)C(F)(F)F)C(=O)C1CCS(=O)(=O)CC1 |r| Show InChI InChI=1S/C27H25F8NO5S2/c28-19-3-5-20(6-4-19)43(40,41)24-11-12-36(23(37)16-9-13-42(38,39)14-10-16)22(24)8-1-17-15-18(2-7-21(17)24)25(29,26(30,31)32)27(33,34)35/h2-7,15-16,22H,1,8-14H2/t22-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00112
BindingDB Entry DOI: 10.7270/Q21R6V6T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50557811

(CHEMBL4779994)Show SMILES [H][C@@]12CCc3cc(ccc3[C@@]1(CC[C@H]2NC(=O)[C@](C)(O)CS(C)(=O)=O)S(=O)(=O)c1ccc(F)cc1)C(F)(C(F)(F)F)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00112
BindingDB Entry DOI: 10.7270/Q21R6V6T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50557810

(CHEMBL4741364)Show SMILES [H][C@@]12CCc3cc(ccc3[C@@]1(CC[C@H]2NC(=O)[C@@](C)(O)CS(C)(=O)=O)S(=O)(=O)c1ccc(F)cc1)C(F)(C(F)(F)F)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00112
BindingDB Entry DOI: 10.7270/Q21R6V6T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50557807

(CHEMBL4744956)Show SMILES [H][C@@]12CCc3nc(ccc3[C@@]1(CC[C@H]2NC(=O)C1CCS(=O)(=O)CC1)S(=O)(=O)c1ccc(F)cc1)C(F)(C(F)(F)F)C(F)(F)F |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00112
BindingDB Entry DOI: 10.7270/Q21R6V6T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50557799
(CHEMBL4750603)Show SMILES [H][C@@]12CCc3nc(ccc3[C@@]1(CCN2C(=O)C1CCS(=O)(=O)CC1)S(=O)(=O)c1ccc(F)cc1)C(F)(C(F)(F)F)C(F)(F)F |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00112
BindingDB Entry DOI: 10.7270/Q21R6V6T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50557806

(CHEMBL4800380)Show SMILES [H][C@@]12CCc3nc(ccc3[C@@]1(CC[C@H]2NC(=O)CC(C)(C)O)S(=O)(=O)c1ccc(F)cc1)C(F)(C(F)(F)F)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00112
BindingDB Entry DOI: 10.7270/Q21R6V6T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50557809

(CHEMBL4760466)Show SMILES [H][C@@]12CCc3nc(ccc3[C@@]1(CC[C@H]2NC(=O)[C@](C)(O)CS(C)(=O)=O)S(=O)(=O)c1ccc(F)cc1)C(F)(C(F)(F)F)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00112
BindingDB Entry DOI: 10.7270/Q21R6V6T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50557800
(CHEMBL4754719)Show SMILES [H][C@@]12CCc3nc(ccc3[C@@]1(CCN2C(=O)[C@@H]1CCC(=O)N1CCC#N)S(=O)(=O)c1ccc(F)cc1)C(F)(C(F)(F)F)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00112
BindingDB Entry DOI: 10.7270/Q21R6V6T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50557810

(CHEMBL4741364)Show SMILES [H][C@@]12CCc3cc(ccc3[C@@]1(CC[C@H]2NC(=O)[C@@](C)(O)CS(C)(=O)=O)S(=O)(=O)c1ccc(F)cc1)C(F)(C(F)(F)F)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00112
BindingDB Entry DOI: 10.7270/Q21R6V6T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50557809

(CHEMBL4760466)Show SMILES [H][C@@]12CCc3nc(ccc3[C@@]1(CC[C@H]2NC(=O)[C@](C)(O)CS(C)(=O)=O)S(=O)(=O)c1ccc(F)cc1)C(F)(C(F)(F)F)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00112
BindingDB Entry DOI: 10.7270/Q21R6V6T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50557807

(CHEMBL4744956)Show SMILES [H][C@@]12CCc3nc(ccc3[C@@]1(CC[C@H]2NC(=O)C1CCS(=O)(=O)CC1)S(=O)(=O)c1ccc(F)cc1)C(F)(C(F)(F)F)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00112
BindingDB Entry DOI: 10.7270/Q21R6V6T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50557805

(CHEMBL4750756)Show SMILES [H][C@@]12CCc3nc(ccc3[C@@]1(CC[C@H]2NC(=O)C(C)(C)O)S(=O)(=O)c1ccc(F)cc1)C(F)(C(F)(F)F)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00112
BindingDB Entry DOI: 10.7270/Q21R6V6T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50557804
(CHEMBL4793083)Show SMILES [H][C@@]12CCc3cc(cnc3[C@@]1(CCN2C(=O)[C@@H]1CCC(=O)N1CCC#N)S(=O)(=O)c1ccc(F)cc1)C(F)(C(F)(F)F)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00112
BindingDB Entry DOI: 10.7270/Q21R6V6T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50557799

(CHEMBL4750603)Show SMILES [H][C@@]12CCc3nc(ccc3[C@@]1(CCN2C(=O)C1CCS(=O)(=O)CC1)S(=O)(=O)c1ccc(F)cc1)C(F)(C(F)(F)F)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00112
BindingDB Entry DOI: 10.7270/Q21R6V6T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM414262

(US10435369, Example 149)Show SMILES Fc1ccc(cc1)S(=O)(=O)[C@@]12CC[C@@H](NC(=O)C3CCS(=O)(=O)CC3)[C@@H]1CCc1cc(ccc21)C(F)(C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C28H27F8NO5S2/c29-19-3-5-20(6-4-19)44(41,42)25-12-9-23(37-24(38)16-10-13-43(39,40)14-11-16)22(25)7-1-17-15-18(2-8-21(17)25)26(30,27(31,32)33)28(34,35)36/h2-6,8,15-16,22-23H,1,7,9-14H2,(H,37,38)/t22-,23+,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00112
BindingDB Entry DOI: 10.7270/Q21R6V6T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50557807

(CHEMBL4744956)Show SMILES [H][C@@]12CCc3nc(ccc3[C@@]1(CC[C@H]2NC(=O)C1CCS(=O)(=O)CC1)S(=O)(=O)c1ccc(F)cc1)C(F)(C(F)(F)F)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00112
BindingDB Entry DOI: 10.7270/Q21R6V6T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50557805

(CHEMBL4750756)Show SMILES [H][C@@]12CCc3nc(ccc3[C@@]1(CC[C@H]2NC(=O)C(C)(C)O)S(=O)(=O)c1ccc(F)cc1)C(F)(C(F)(F)F)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00112
BindingDB Entry DOI: 10.7270/Q21R6V6T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50557809

(CHEMBL4760466)Show SMILES [H][C@@]12CCc3nc(ccc3[C@@]1(CC[C@H]2NC(=O)[C@](C)(O)CS(C)(=O)=O)S(=O)(=O)c1ccc(F)cc1)C(F)(C(F)(F)F)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00112
BindingDB Entry DOI: 10.7270/Q21R6V6T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50557804

(CHEMBL4793083)Show SMILES [H][C@@]12CCc3cc(cnc3[C@@]1(CCN2C(=O)[C@@H]1CCC(=O)N1CCC#N)S(=O)(=O)c1ccc(F)cc1)C(F)(C(F)(F)F)C(F)(F)F |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00112
BindingDB Entry DOI: 10.7270/Q21R6V6T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50557802

(CHEMBL4748187)Show SMILES [H][C@@]12CCc3cc(ncc3[C@@]1(CCN2C(=O)[C@@H]1CCC(=O)N1CCC#N)S(=O)(=O)c1ccc(F)cc1)C(F)(C(F)(F)F)C(F)(F)F |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00112
BindingDB Entry DOI: 10.7270/Q21R6V6T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50557811

(CHEMBL4779994)Show SMILES [H][C@@]12CCc3cc(ccc3[C@@]1(CC[C@H]2NC(=O)[C@](C)(O)CS(C)(=O)=O)S(=O)(=O)c1ccc(F)cc1)C(F)(C(F)(F)F)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00112
BindingDB Entry DOI: 10.7270/Q21R6V6T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50557800

(CHEMBL4754719)Show SMILES [H][C@@]12CCc3nc(ccc3[C@@]1(CCN2C(=O)[C@@H]1CCC(=O)N1CCC#N)S(=O)(=O)c1ccc(F)cc1)C(F)(C(F)(F)F)C(F)(F)F |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00112
BindingDB Entry DOI: 10.7270/Q21R6V6T |
More data for this
Ligand-Target Pair | |
Protein kinase C
(Mus musculus) | BDBM50092014

(Butyric acid (1aS,1bS,4aS,7aS,8R,9S,9aR)-9-butyryl...)Show SMILES CCCC(=O)O[C@@H]1[C@@H](C)[C@]2(O)[C@@H]3C=C(C)C(=O)[C@]3(O)CC(CO)=C[C@H]2[C@@H]2C(C)(C)[C@]12OC(=O)CCC |r,c:23,t:12| Show InChI InChI=1S/C28H40O8/c1-7-9-20(30)35-24-16(4)27(34)18(22-25(5,6)28(22,24)36-21(31)10-8-2)12-17(14-29)13-26(33)19(27)11-15(3)23(26)32/h11-12,16,18-19,22,24,29,33-34H,7-10,13-14H2,1-6H3/t16-,18+,19-,22?,24-,26+,27-,28-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
| |
ACS Chem Biol 8: 767-77 (2013)
Article DOI: 10.1021/cb300671s
BindingDB Entry DOI: 10.7270/Q20V8BF7 |
More data for this
Ligand-Target Pair | |
Protein kinase C
(Mus musculus) | BDBM50258529

(CHEMBL449158 | bryostatin 1)Show SMILES CCC\C=C\C=C\C(=O)O[C@H]1\C(C[C@H]2C[C@@H](OC(=O)C[C@H](O)C[C@@H]3C[C@H](OC(C)=O)C(C)(C)[C@](O)(C[C@@H]4C\C(C[C@@H](O4)\C=C\C(C)(C)[C@]1(O)O2)=C\C(=O)OC)O3)[C@@H](C)O)=C\C(=O)OC |r,t:43| Show InChI InChI=1S/C47H68O17/c1-10-11-12-13-14-15-39(51)62-43-31(22-41(53)58-9)21-34-25-37(28(2)48)61-42(54)24-32(50)23-35-26-38(59-29(3)49)45(6,7)46(55,63-35)27-36-19-30(20-40(52)57-8)18-33(60-36)16-17-44(4,5)47(43,56)64-34/h12-17,20,22,28,32-38,43,48,50,55-56H,10-11,18-19,21,23-27H2,1-9H3/b13-12+,15-14+,17-16+,30-20+,31-22+/t28-,32-,33+,34+,35-,36+,37-,38+,43+,46+,47-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
| |
ACS Chem Biol 8: 767-77 (2013)
Article DOI: 10.1021/cb300671s
BindingDB Entry DOI: 10.7270/Q20V8BF7 |
More data for this
Ligand-Target Pair | |
Protein kinase C
(Mus musculus) | BDBM102423
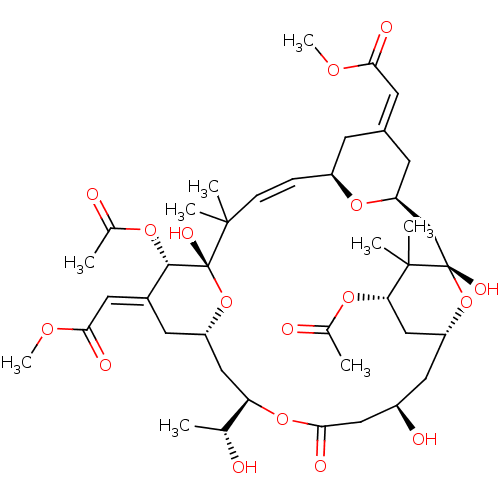
(Bryostatin | Bryostatin 7)Show SMILES COC(=O)\C=C1\C[C@H]2C[C@]3(O)O[C@@H](C[C@H](OC(C)=O)C3(C)C)C[C@@H](O)CC(=O)O[C@H](C[C@@H]3C\C(=C/C(=O)OC)[C@H](OC(C)=O)[C@@](O)(O3)C(C)(C)\C=C\[C@@H](C1)O2)[C@@H](C)O |t:52| Show InChI InChI=1S/C41H60O17/c1-22(42)32-19-29-15-26(16-35(47)52-9)37(54-24(3)44)41(50,58-29)38(4,5)11-10-28-12-25(14-34(46)51-8)13-31(55-28)21-40(49)39(6,7)33(53-23(2)43)20-30(57-40)17-27(45)18-36(48)56-32/h10-11,14,16,22,27-33,37,42,45,49-50H,12-13,15,17-21H2,1-9H3/b11-10+,25-14+,26-16+/t22-,27-,28+,29+,30-,31+,32-,33+,37+,40+,41-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
| |
ACS Chem Biol 8: 767-77 (2013)
Article DOI: 10.1021/cb300671s
BindingDB Entry DOI: 10.7270/Q20V8BF7 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50092014

(Butyric acid (1aS,1bS,4aS,7aS,8R,9S,9aR)-9-butyryl...)Show SMILES CCCC(=O)O[C@@H]1[C@@H](C)[C@]2(O)[C@@H]3C=C(C)C(=O)[C@]3(O)CC(CO)=C[C@H]2[C@@H]2C(C)(C)[C@]12OC(=O)CCC |r,c:23,t:12| Show InChI InChI=1S/C28H40O8/c1-7-9-20(30)35-24-16(4)27(34)18(22-25(5,6)28(22,24)36-21(31)10-8-2)12-17(14-29)13-26(33)19(27)11-15(3)23(26)32/h11-12,16,18-19,22,24,29,33-34H,7-10,13-14H2,1-6H3/t16-,18+,19-,22?,24-,26+,27-,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
| |
ACS Chem Biol 8: 767-77 (2013)
Article DOI: 10.1021/cb300671s
BindingDB Entry DOI: 10.7270/Q20V8BF7 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50258529

(CHEMBL449158 | bryostatin 1)Show SMILES CCC\C=C\C=C\C(=O)O[C@H]1\C(C[C@H]2C[C@@H](OC(=O)C[C@H](O)C[C@@H]3C[C@H](OC(C)=O)C(C)(C)[C@](O)(C[C@@H]4C\C(C[C@@H](O4)\C=C\C(C)(C)[C@]1(O)O2)=C\C(=O)OC)O3)[C@@H](C)O)=C\C(=O)OC |r,t:43| Show InChI InChI=1S/C47H68O17/c1-10-11-12-13-14-15-39(51)62-43-31(22-41(53)58-9)21-34-25-37(28(2)48)61-42(54)24-32(50)23-35-26-38(59-29(3)49)45(6,7)46(55,63-35)27-36-19-30(20-40(52)57-8)18-33(60-36)16-17-44(4,5)47(43,56)64-34/h12-17,20,22,28,32-38,43,48,50,55-56H,10-11,18-19,21,23-27H2,1-9H3/b13-12+,15-14+,17-16+,30-20+,31-22+/t28-,32-,33+,34+,35-,36+,37-,38+,43+,46+,47-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
| |
ACS Chem Biol 8: 767-77 (2013)
Article DOI: 10.1021/cb300671s
BindingDB Entry DOI: 10.7270/Q20V8BF7 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM102423

(Bryostatin | Bryostatin 7)Show SMILES COC(=O)\C=C1\C[C@H]2C[C@]3(O)O[C@@H](C[C@H](OC(C)=O)C3(C)C)C[C@@H](O)CC(=O)O[C@H](C[C@@H]3C\C(=C/C(=O)OC)[C@H](OC(C)=O)[C@@](O)(O3)C(C)(C)\C=C\[C@@H](C1)O2)[C@@H](C)O |t:52| Show InChI InChI=1S/C41H60O17/c1-22(42)32-19-29-15-26(16-35(47)52-9)37(54-24(3)44)41(50,58-29)38(4,5)11-10-28-12-25(14-34(46)51-8)13-31(55-28)21-40(49)39(6,7)33(53-23(2)43)20-30(57-40)17-27(45)18-36(48)56-32/h10-11,14,16,22,27-33,37,42,45,49-50H,12-13,15,17-21H2,1-9H3/b11-10+,25-14+,26-16+/t22-,27-,28+,29+,30-,31+,32-,33+,37+,40+,41-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
| |
ACS Chem Biol 8: 767-77 (2013)
Article DOI: 10.1021/cb300671s
BindingDB Entry DOI: 10.7270/Q20V8BF7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data