

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50129580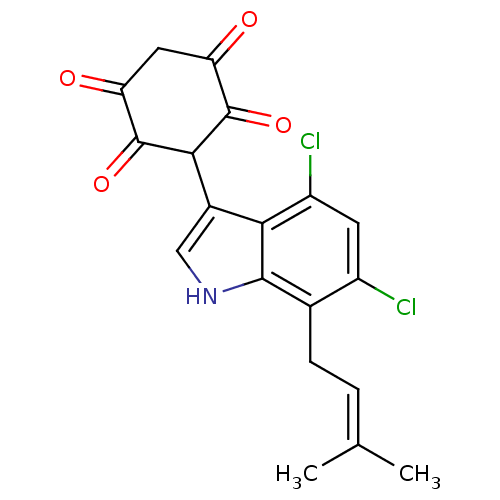 (3-[4,6-Dichloro-7-(3-methyl-but-2-enyl)-1H-indol-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Inhibitory constant of compound against Cell division cycle 25B was determined using mFP as a substrate | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50129574 (2,5-Dihydroxy-3-[7-(3-methyl-but-2-enyl)-1H-indol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Inhibitory constant of compound against Cell division cycle 25B was determined using mFP as a substrate | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 3 (Homo sapiens (Human)) | BDBM50129576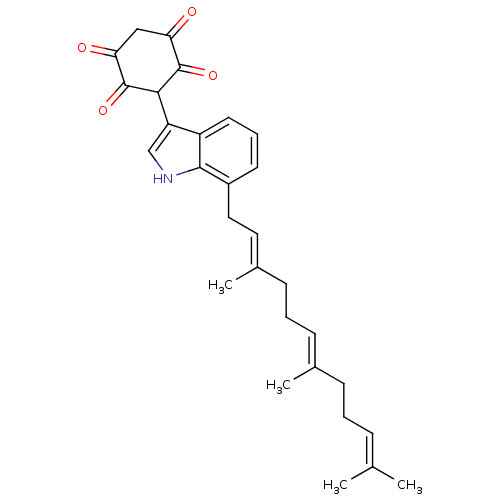 (2,5-Dihydroxy-3-[7-(3,7,11-trimethyl-dodeca-2,6,10...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Inhibitory constant of compound against Cell division cycle 25 was determined | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50129575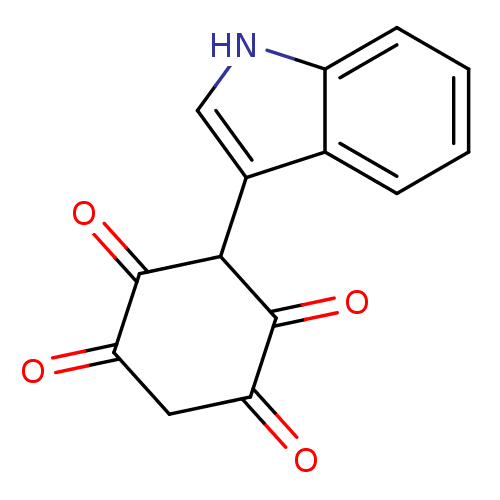 (2,5-Dihydroxy-3-(1H-indol-3-yl)-[1,4]benzoquinone ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Inhibitory constant of compound against Cdc25B phosphatase was determined using mFP as a substrate | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50207432 (CHEMBL3909479) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Beni-Suef University Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as reduction in oxidation of TMPD using arachidonic acid as substrate preincubated for 5 mins followed by substrat... | Bioorg Med Chem 25: 665-676 (2017) Article DOI: 10.1016/j.bmc.2016.11.037 BindingDB Entry DOI: 10.7270/Q2542QKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Beni-Suef University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 assessed as reduction in oxidation of TMPD using arachidonic acid as substrate preincubated for 5 mins followed... | Bioorg Med Chem 25: 665-676 (2017) Article DOI: 10.1016/j.bmc.2016.11.037 BindingDB Entry DOI: 10.7270/Q2542QKM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50565488 (CHEMBL4783386) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using epoxy flour 7 as substrate measured after 30 mins by cell-based fluorescence assay | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112662 BindingDB Entry DOI: 10.7270/Q2B85CWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50207431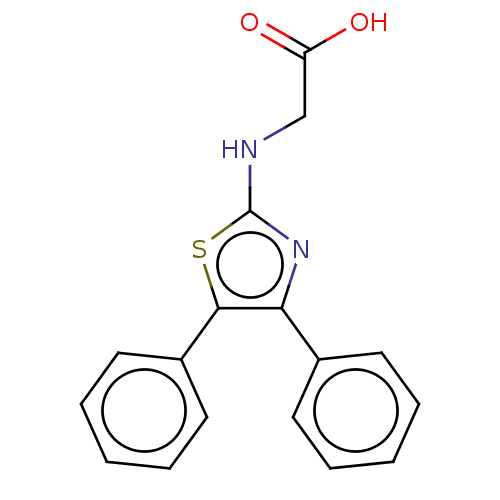 (CHEMBL3892572) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Beni-Suef University Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as reduction in oxidation of TMPD using arachidonic acid as substrate preincubated for 5 mins followed by substrat... | Bioorg Med Chem 25: 665-676 (2017) Article DOI: 10.1016/j.bmc.2016.11.037 BindingDB Entry DOI: 10.7270/Q2542QKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM25737 (12-[(adamantan-1-ylcarbamoyl)amino]dodecanoic acid...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using epoxy flour 7 as substrate measured after 30 mins by cell-based fluorescence assay | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112662 BindingDB Entry DOI: 10.7270/Q2B85CWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50207435 (CHEMBL3960755) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Beni-Suef University Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as reduction in oxidation of TMPD using arachidonic acid as substrate preincubated for 5 mins followed by substrat... | Bioorg Med Chem 25: 665-676 (2017) Article DOI: 10.1016/j.bmc.2016.11.037 BindingDB Entry DOI: 10.7270/Q2542QKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50565490 (CHEMBL4788670) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using epoxy flour 7 as substrate measured after 30 mins by cell-based fluorescence assay | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112662 BindingDB Entry DOI: 10.7270/Q2B85CWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50207430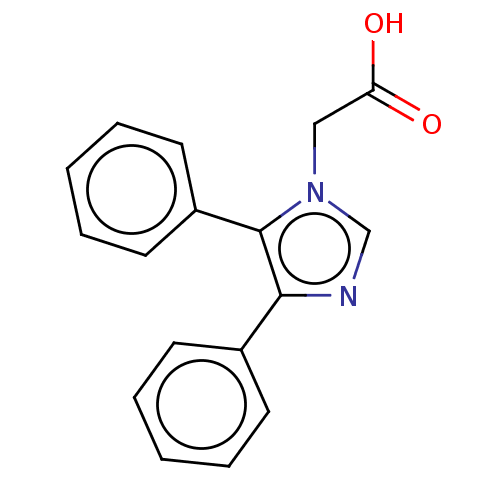 (CHEMBL3924357) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
Beni-Suef University Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as reduction in oxidation of TMPD using arachidonic acid as substrate preincubated for 5 mins followed by substrat... | Bioorg Med Chem 25: 665-676 (2017) Article DOI: 10.1016/j.bmc.2016.11.037 BindingDB Entry DOI: 10.7270/Q2542QKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
Beni-Suef University Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as reduction in oxidation of TMPD using arachidonic acid as substrate preincubated for 5 mins followed by substrat... | Bioorg Med Chem 25: 665-676 (2017) Article DOI: 10.1016/j.bmc.2016.11.037 BindingDB Entry DOI: 10.7270/Q2542QKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human COX2 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition and measured after 2 ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112662 BindingDB Entry DOI: 10.7270/Q2B85CWK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50565489 (CHEMBL4779788) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using epoxy flour 7 as substrate measured after 30 mins by cell-based fluorescence assay | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112662 BindingDB Entry DOI: 10.7270/Q2B85CWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50129579 (2,5-Dihydroxy-3-[7-(2-methyl-benzyl)-1H-indol-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Eight point inhibitory concentration against Cell division cycle 25A was determined | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50129579 (2,5-Dihydroxy-3-[7-(2-methyl-benzyl)-1H-indol-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Eight point inhibitory concentration against Cell division cycle 25B was determined | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50129583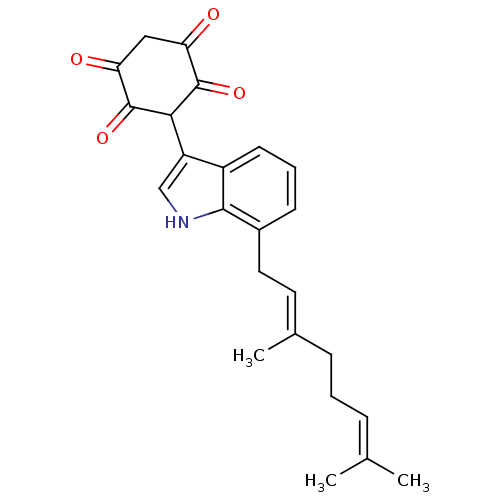 (3-[7-(3,7-Dimethyl-octa-2,6-dienyl)-1H-indol-3-yl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Eight point inhibitory concentration against Cell division cycle 25A was determined | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50565483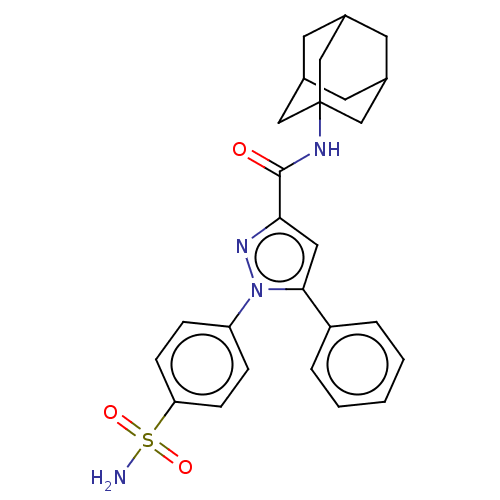 (CHEMBL4790503) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using epoxy flour 7 as substrate measured after 30 mins by cell-based fluorescence assay | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112662 BindingDB Entry DOI: 10.7270/Q2B85CWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50565494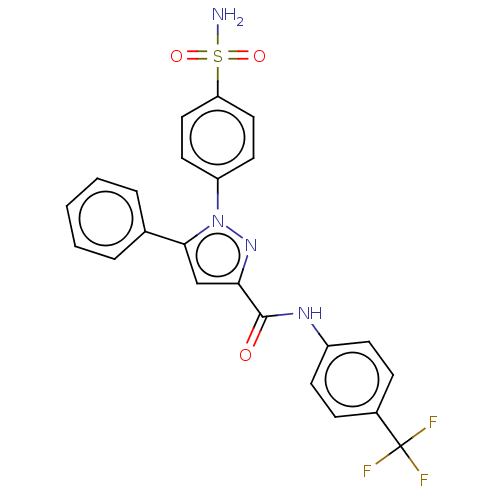 (CHEMBL4799379) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using epoxy flour 7 as substrate measured after 30 mins by cell-based fluorescence assay | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112662 BindingDB Entry DOI: 10.7270/Q2B85CWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50565488 (CHEMBL4783386) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human COX2 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition and measured after 2 ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112662 BindingDB Entry DOI: 10.7270/Q2B85CWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50565486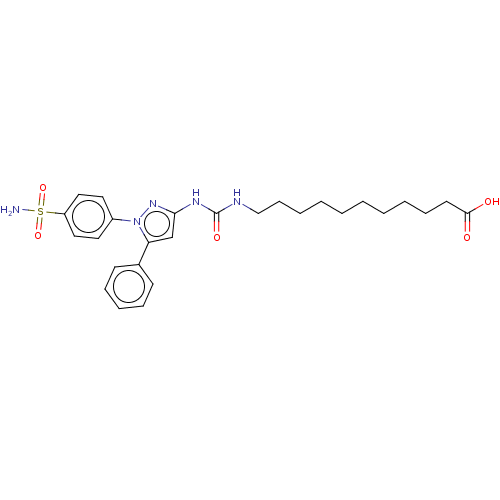 (CHEMBL4789344) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using epoxy flour 7 as substrate measured after 30 mins by cell-based fluorescence assay | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112662 BindingDB Entry DOI: 10.7270/Q2B85CWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50565493 (CHEMBL4799227) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using epoxy flour 7 as substrate measured after 30 mins by cell-based fluorescence assay | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112662 BindingDB Entry DOI: 10.7270/Q2B85CWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50565487 (CHEMBL4790061) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using epoxy flour 7 as substrate measured after 30 mins by cell-based fluorescence assay | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112662 BindingDB Entry DOI: 10.7270/Q2B85CWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50565491 (CHEMBL4789520) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using epoxy flour 7 as substrate measured after 30 mins by cell-based fluorescence assay | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112662 BindingDB Entry DOI: 10.7270/Q2B85CWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50565490 (CHEMBL4788670) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human COX2 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition and measured after 2 ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112662 BindingDB Entry DOI: 10.7270/Q2B85CWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50565485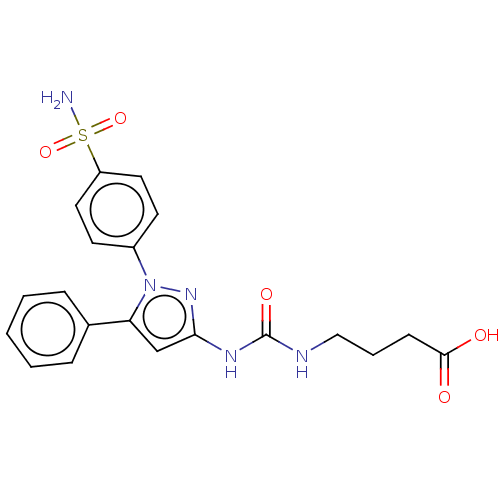 (CHEMBL4792522) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human COX2 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition and measured after 2 ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112662 BindingDB Entry DOI: 10.7270/Q2B85CWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50565489 (CHEMBL4779788) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human COX2 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition and measured after 2 ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112662 BindingDB Entry DOI: 10.7270/Q2B85CWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50565494 (CHEMBL4799379) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human COX2 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition and measured after 2 ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112662 BindingDB Entry DOI: 10.7270/Q2B85CWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50565483 (CHEMBL4790503) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human COX2 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition and measured after 2 ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112662 BindingDB Entry DOI: 10.7270/Q2B85CWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50565486 (CHEMBL4789344) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human COX2 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition and measured after 2 ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112662 BindingDB Entry DOI: 10.7270/Q2B85CWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50129585 (3-(7-Benzyl-1H-indol-3-yl)-2,5-dihydroxy-[1,4]benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Eight point inhibitory concentration against Cell division cycle 25A was determined | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50129585 (3-(7-Benzyl-1H-indol-3-yl)-2,5-dihydroxy-[1,4]benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Eight point inhibitory concentration against Cell division cycle 25B was determined | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50129580 (3-[4,6-Dichloro-7-(3-methyl-but-2-enyl)-1H-indol-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Eight point inhibitory concentration against Cell division cycle 25B was determined | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50565484 (CHEMBL4777518) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using epoxy flour 7 as substrate measured after 30 mins by cell-based fluorescence assay | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112662 BindingDB Entry DOI: 10.7270/Q2B85CWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50129576 (2,5-Dihydroxy-3-[7-(3,7,11-trimethyl-dodeca-2,6,10...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Eight point inhibitory concentration against Cell division cycle 25A was determined | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50129576 (2,5-Dihydroxy-3-[7-(3,7,11-trimethyl-dodeca-2,6,10...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Eight point inhibitory concentration against Cell division cycle 25B was determined | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50565484 (CHEMBL4777518) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human COX2 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition and measured after 2 ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112662 BindingDB Entry DOI: 10.7270/Q2B85CWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50129572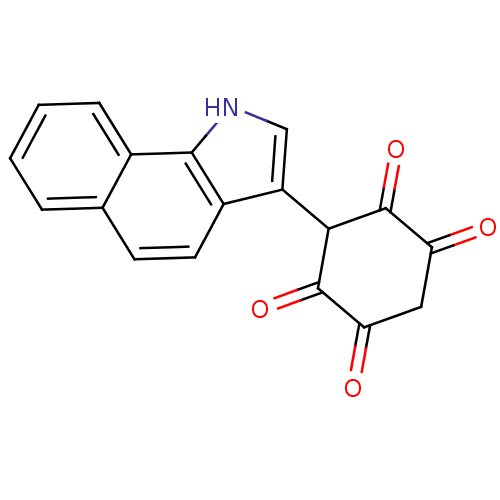 (3-(1H-Benzo[g]indol-3-yl)-2,5-dihydroxy-[1,4]benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Eight point inhibitory concentration against Cell division cycle 25A was determined | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50129577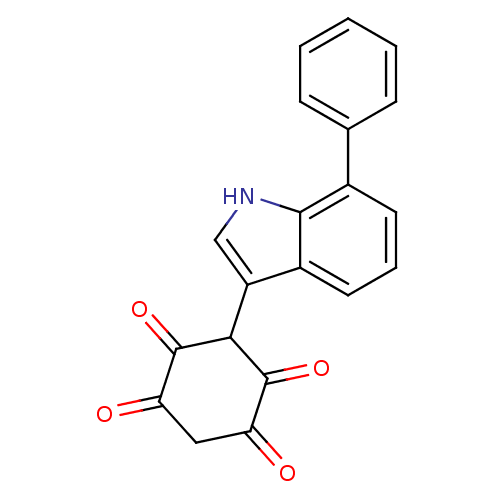 (2,5-Dihydroxy-3-(7-phenyl-1H-indol-3-yl)-[1,4]benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Eight point inhibitory concentration against Cell division cycle 25A was determined | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50129577 (2,5-Dihydroxy-3-(7-phenyl-1H-indol-3-yl)-[1,4]benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Eight point inhibitory concentration against Cell division cycle 25B was determined | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50207425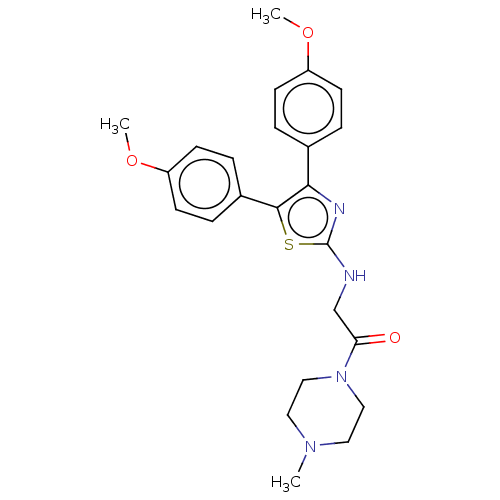 (CHEMBL3935079) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beni-Suef University Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as reduction in oxidation of TMPD using arachidonic acid as substrate preincubated for 5 mins followed by substrat... | Bioorg Med Chem 25: 665-676 (2017) Article DOI: 10.1016/j.bmc.2016.11.037 BindingDB Entry DOI: 10.7270/Q2542QKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50565487 (CHEMBL4790061) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human COX2 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition and measured after 2 ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112662 BindingDB Entry DOI: 10.7270/Q2B85CWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50207434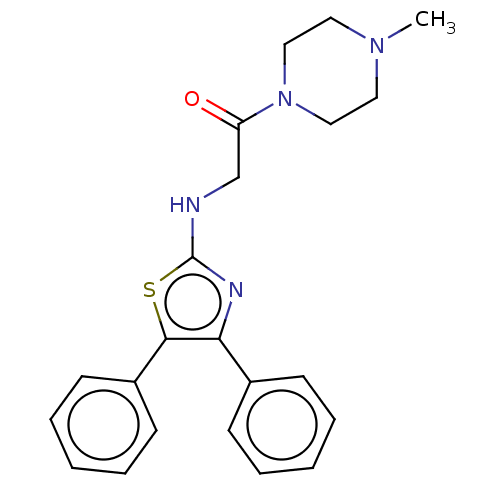 (CHEMBL3981625) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beni-Suef University Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as reduction in oxidation of TMPD using arachidonic acid as substrate preincubated for 5 mins followed by substrat... | Bioorg Med Chem 25: 665-676 (2017) Article DOI: 10.1016/j.bmc.2016.11.037 BindingDB Entry DOI: 10.7270/Q2542QKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50565492 (CHEMBL4794910) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human COX2 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition and measured after 2 ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112662 BindingDB Entry DOI: 10.7270/Q2B85CWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50207428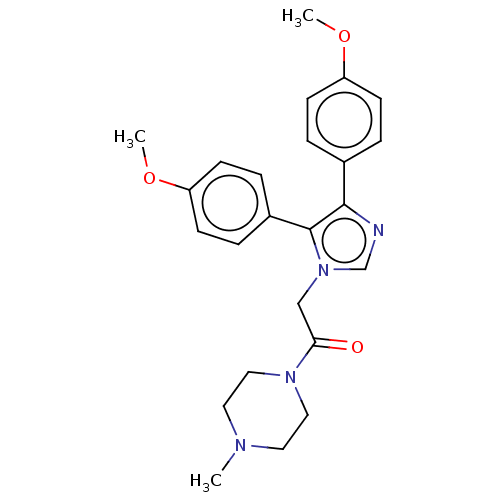 (CHEMBL3952057) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beni-Suef University Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as reduction in oxidation of TMPD using arachidonic acid as substrate preincubated for 5 mins followed by substrat... | Bioorg Med Chem 25: 665-676 (2017) Article DOI: 10.1016/j.bmc.2016.11.037 BindingDB Entry DOI: 10.7270/Q2542QKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50565492 (CHEMBL4794910) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using epoxy flour 7 as substrate measured after 30 mins by cell-based fluorescence assay | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112662 BindingDB Entry DOI: 10.7270/Q2B85CWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 3 (Homo sapiens (Human)) | BDBM50129579 (2,5-Dihydroxy-3-[7-(2-methyl-benzyl)-1H-indol-3-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Eight point inhibitory concentration against Cell division cycle 25 degree C was determined | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 3 (Homo sapiens (Human)) | BDBM50129576 (2,5-Dihydroxy-3-[7-(3,7,11-trimethyl-dodeca-2,6,10...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Eight point inhibitory concentration against Cell division cycle 25 degree C was determined | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50129574 (2,5-Dihydroxy-3-[7-(3-methyl-but-2-enyl)-1H-indol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Inhibitory concentration of compound against mutant M532A of Cell division cycle 25B was determined | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 152 total ) | Next | Last >> |