 Found 46 hits with Last Name = 'scampuddu' and Initial = 'a'
Found 46 hits with Last Name = 'scampuddu' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 2
(MOUSE) | BDBM50200169
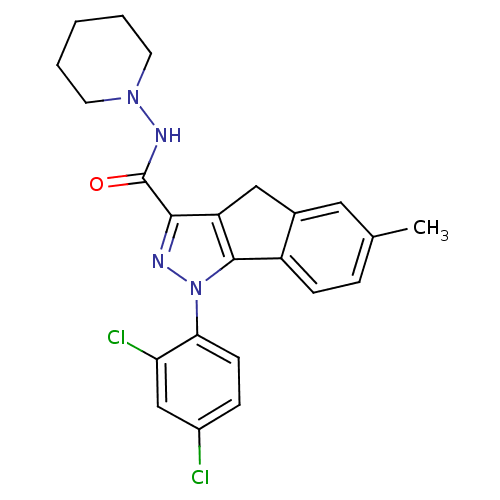
(6-methyl-1-(2',4'-dichlorophenyl)-N-piperidin-1-yl...)Show SMILES Cc1ccc-2c(Cc3c(nn(c-23)-c2ccc(Cl)cc2Cl)C(=O)NN2CCCCC2)c1 Show InChI InChI=1S/C23H22Cl2N4O/c1-14-5-7-17-15(11-14)12-18-21(23(30)27-28-9-3-2-4-10-28)26-29(22(17)18)20-8-6-16(24)13-19(20)25/h5-8,11,13H,2-4,9-10,12H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from CB2 receptor in CD-1 mouse spleen homogenate after 1 hr by liquid scintillation counting method |
Bioorg Med Chem 24: 5291-5301 (2016)
Article DOI: 10.1016/j.bmc.2016.08.055
BindingDB Entry DOI: 10.7270/Q2HT2SV1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50180022
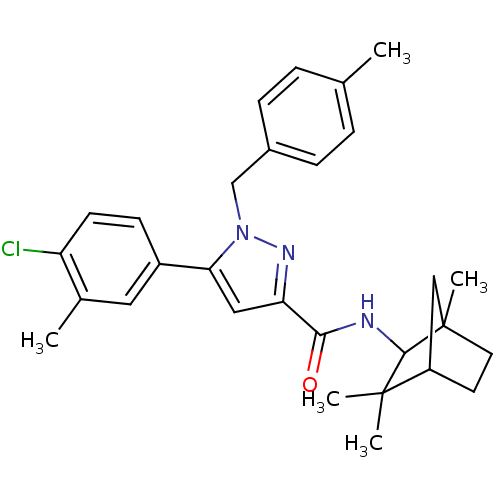
(5-(4-chloro-3-methyl-phenyl)-1-(4-methyl-benzyl)-1...)Show SMILES Cc1ccc(Cn2nc(cc2-c2ccc(Cl)c(C)c2)C(=O)NC2C3(C)CCC(C3)C2(C)C)cc1 |THB:21:22:26.25:28| Show InChI InChI=1S/C29H34ClN3O/c1-18-6-8-20(9-7-18)17-33-25(21-10-11-23(30)19(2)14-21)15-24(32-33)26(34)31-27-28(3,4)22-12-13-29(27,5)16-22/h6-11,14-15,22,27H,12-13,16-17H2,1-5H3,(H,31,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from human frontal cortex CB2 receptor expressed in CHO cell membrane after 1 hr by scintillation counting method |
Bioorg Med Chem 24: 5291-5301 (2016)
Article DOI: 10.1016/j.bmc.2016.08.055
BindingDB Entry DOI: 10.7270/Q2HT2SV1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM82141

(WIN 55212-2)Show SMILES CC1=C(C2C=CC=C3OC[C@@H](CN4CCOCC4)N1C23)C(=O)c1cccc2ccccc12 |r,c:4,t:1,6| Show InChI InChI=1S/C27H28N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20,23,26H,12-17H2,1H3/t20-,23?,26?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from human CB2 receptor expressed in CHO cell membrane after 60 mins by scintillation counting method |
Bioorg Med Chem 24: 5291-5301 (2016)
Article DOI: 10.1016/j.bmc.2016.08.055
BindingDB Entry DOI: 10.7270/Q2HT2SV1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM82141

(WIN 55212-2)Show SMILES CC1=C(C2C=CC=C3OC[C@@H](CN4CCOCC4)N1C23)C(=O)c1cccc2ccccc12 |r,c:4,t:1,6| Show InChI InChI=1S/C27H28N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20,23,26H,12-17H2,1H3/t20-,23?,26?/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from human CB1 receptor expressed in CHO cell membrane after 90 mins by scintillation counting method |
Bioorg Med Chem 24: 5291-5301 (2016)
Article DOI: 10.1016/j.bmc.2016.08.055
BindingDB Entry DOI: 10.7270/Q2HT2SV1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50534085

(CHEMBL4458732)Show SMILES Cc1ccc(cc1)-c1cc2ncccc2c2cc(nn12)C(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:26:27:24.25.30:31,THB:26:25:31:32.27.28,28:27:24:30.29.31,28:29:24:32.26.27| Show InChI InChI=1S/C28H28N4O/c1-17-4-6-21(7-5-17)25-12-23-22(3-2-8-29-23)26-13-24(31-32(25)26)27(33)30-28-14-18-9-19(15-28)11-20(10-18)16-28/h2-8,12-13,18-20H,9-11,14-16H2,1H3,(H,30,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from human CB2 receptor expressed in CHO cell membrane after 60 mins by scintillation counting method |
Bioorg Med Chem 24: 5291-5301 (2016)
Article DOI: 10.1016/j.bmc.2016.08.055
BindingDB Entry DOI: 10.7270/Q2HT2SV1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM60994

((10R,10aR)-6,6,9-Trimethyl-3-pentyl-6a,7,8,10a-tet...)Show SMILES CCCCCc1cc(O)c2[C@@H]3C=C(C)CC[C@H]3C(C)(C)Oc2c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-15-12-18(22)20-16-11-14(2)9-10-17(16)21(3,4)23-19(20)13-15/h11-13,16-17,22H,5-10H2,1-4H3/t16-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor (unknown origin) |
Bioorg Med Chem 24: 5291-5301 (2016)
Article DOI: 10.1016/j.bmc.2016.08.055
BindingDB Entry DOI: 10.7270/Q2HT2SV1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM60994

((10R,10aR)-6,6,9-Trimethyl-3-pentyl-6a,7,8,10a-tet...)Show SMILES CCCCCc1cc(O)c2[C@@H]3C=C(C)CC[C@H]3C(C)(C)Oc2c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-15-12-18(22)20-16-11-14(2)9-10-17(16)21(3,4)23-19(20)13-15/h11-13,16-17,22H,5-10H2,1-4H3/t16-,17-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Binding affinity to CB1 receptor (unknown origin) |
Bioorg Med Chem 24: 5291-5301 (2016)
Article DOI: 10.1016/j.bmc.2016.08.055
BindingDB Entry DOI: 10.7270/Q2HT2SV1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50534088

(CHEMBL4453164)Show SMILES Cc1ccc(cc1)-c1cc2ncccc2c2cc(nn12)C(=O)N[C@H]1CC2CCC1(C)C2(C)C |r,TLB:22:23:27.26:30| Show InChI InChI=1S/C28H30N4O/c1-17-7-9-18(10-8-17)23-15-21-20(6-5-13-29-21)24-16-22(31-32(23)24)26(33)30-25-14-19-11-12-28(25,4)27(19,2)3/h5-10,13,15-16,19,25H,11-12,14H2,1-4H3,(H,30,33)/t19?,25-,28?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from human CB2 receptor expressed in CHO cell membrane after 60 mins by scintillation counting method |
Bioorg Med Chem 24: 5291-5301 (2016)
Article DOI: 10.1016/j.bmc.2016.08.055
BindingDB Entry DOI: 10.7270/Q2HT2SV1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50534082

(CHEMBL4448088)Show SMILES CC1(C)C2CC1[C@H](CNC(=O)c1cc3c4cccnc4cc(-c4ccc(Cl)cc4Cl)n3n1)CC2 |r,TLB:7:6:1:4| Show InChI InChI=1S/C27H26Cl2N4O/c1-27(2)16-6-5-15(20(27)10-16)14-31-26(34)23-13-25-19-4-3-9-30-22(19)12-24(33(25)32-23)18-8-7-17(28)11-21(18)29/h3-4,7-9,11-13,15-16,20H,5-6,10,14H2,1-2H3,(H,31,34)/t15-,16?,20?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from human CB2 receptor expressed in CHO cell membrane after 60 mins by scintillation counting method |
Bioorg Med Chem 24: 5291-5301 (2016)
Article DOI: 10.1016/j.bmc.2016.08.055
BindingDB Entry DOI: 10.7270/Q2HT2SV1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM22988

((5Z,8Z,11Z,14Z)-N-(2-hydroxyethyl)icosa-5,8,11,14-...)Show InChI InChI=1S/C22H37NO2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-22(25)23-20-21-24/h6-7,9-10,12-13,15-16,24H,2-5,8,11,14,17-21H2,1H3,(H,23,25)/b7-6-,10-9-,13-12-,16-15- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Binding affinity to CB1 receptor (unknown origin) |
Bioorg Med Chem 24: 5291-5301 (2016)
Article DOI: 10.1016/j.bmc.2016.08.055
BindingDB Entry DOI: 10.7270/Q2HT2SV1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50534091
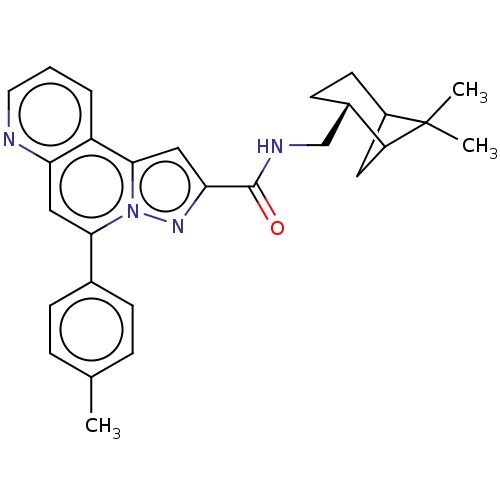
(CHEMBL4525460)Show SMILES Cc1ccc(cc1)-c1cc2ncccc2c2cc(nn12)C(=O)NC[C@@H]1CCC2CC1C2(C)C |r,THB:23:24:30:28| Show InChI InChI=1S/C28H30N4O/c1-17-6-8-18(9-7-17)25-14-23-21(5-4-12-29-23)26-15-24(31-32(25)26)27(33)30-16-19-10-11-20-13-22(19)28(20,2)3/h4-9,12,14-15,19-20,22H,10-11,13,16H2,1-3H3,(H,30,33)/t19-,20?,22?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from human CB2 receptor expressed in CHO cell membrane after 60 mins by scintillation counting method |
Bioorg Med Chem 24: 5291-5301 (2016)
Article DOI: 10.1016/j.bmc.2016.08.055
BindingDB Entry DOI: 10.7270/Q2HT2SV1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50534090

(CHEMBL4528488)Show SMILES Cc1ccc(cc1)-c1cc2ncccc2c2cc(nn12)C(=O)NC1C2CC3CC(C2)CC1C3 |TLB:22:23:25:29.28.27,THB:23:24:27:32.31.30,23:31:25.24.29:27,30:31:25:29.28.27,30:28:25:32.23.31,(14.71,-21.04,;13.39,-20.25,;12.05,-21,;10.72,-20.21,;10.74,-18.67,;12.1,-17.92,;13.41,-18.71,;9.42,-17.89,;8.08,-18.64,;6.75,-17.85,;5.41,-18.6,;4.09,-17.81,;4.11,-16.27,;5.45,-15.52,;6.78,-16.31,;8.12,-15.56,;8.46,-14.06,;10,-13.92,;10.6,-15.33,;9.44,-16.35,;10.78,-12.6,;10.03,-11.24,;12.31,-12.6,;12.7,-11.11,;14.1,-10.54,;15.6,-10.95,;14.4,-9.68,;14.4,-8.2,;13.05,-7.73,;14.1,-8.96,;11.66,-8.31,;11.68,-9.84,;13.07,-10.18,)| Show InChI InChI=1S/C28H28N4O/c1-16-4-6-19(7-5-16)25-14-23-22(3-2-8-29-23)26-15-24(31-32(25)26)28(33)30-27-20-10-17-9-18(12-20)13-21(27)11-17/h2-8,14-15,17-18,20-21,27H,9-13H2,1H3,(H,30,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 133 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from human CB2 receptor expressed in CHO cell membrane after 60 mins by scintillation counting method |
Bioorg Med Chem 24: 5291-5301 (2016)
Article DOI: 10.1016/j.bmc.2016.08.055
BindingDB Entry DOI: 10.7270/Q2HT2SV1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50534089
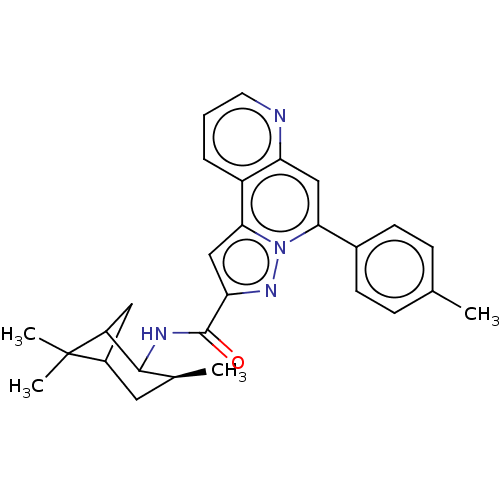
(CHEMBL4549921)Show SMILES C[C@H]1CC2CC(C1NC(=O)c1cc3c4cccnc4cc(-c4ccc(C)cc4)n3n1)C2(C)C |r,TLB:7:6:4:30,THB:0:1:4:30| Show InChI InChI=1S/C28H30N4O/c1-16-7-9-18(10-8-16)24-14-22-20(6-5-11-29-22)25-15-23(31-32(24)25)27(33)30-26-17(2)12-19-13-21(26)28(19,3)4/h5-11,14-15,17,19,21,26H,12-13H2,1-4H3,(H,30,33)/t17-,19?,21?,26?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from human CB2 receptor expressed in CHO cell membrane after 60 mins by scintillation counting method |
Bioorg Med Chem 24: 5291-5301 (2016)
Article DOI: 10.1016/j.bmc.2016.08.055
BindingDB Entry DOI: 10.7270/Q2HT2SV1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50534087
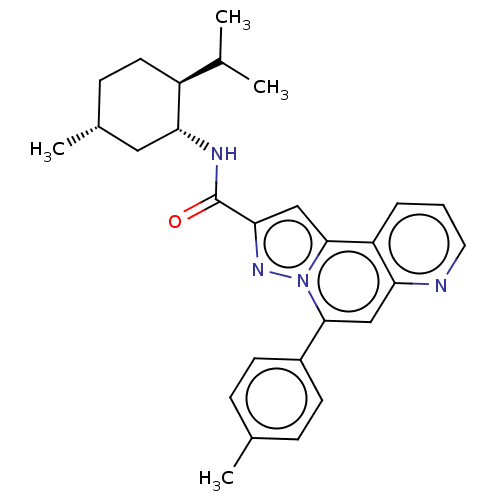
(CHEMBL4520356)Show SMILES CC(C)[C@@H]1CC[C@@H](C)C[C@H]1NC(=O)c1cc2c3cccnc3cc(-c3ccc(C)cc3)n2n1 |r| Show InChI InChI=1S/C28H32N4O/c1-17(2)21-12-9-19(4)14-24(21)30-28(33)25-16-27-22-6-5-13-29-23(22)15-26(32(27)31-25)20-10-7-18(3)8-11-20/h5-8,10-11,13,15-17,19,21,24H,9,12,14H2,1-4H3,(H,30,33)/t19-,21+,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from human CB2 receptor expressed in CHO cell membrane after 60 mins by scintillation counting method |
Bioorg Med Chem 24: 5291-5301 (2016)
Article DOI: 10.1016/j.bmc.2016.08.055
BindingDB Entry DOI: 10.7270/Q2HT2SV1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Mus musculus (Mouse)) | BDBM50200169

(6-methyl-1-(2',4'-dichlorophenyl)-N-piperidin-1-yl...)Show SMILES Cc1ccc-2c(Cc3c(nn(c-23)-c2ccc(Cl)cc2Cl)C(=O)NN2CCCCC2)c1 Show InChI InChI=1S/C23H22Cl2N4O/c1-14-5-7-17-15(11-14)12-18-21(23(30)27-28-9-3-2-4-10-28)26-29(22(17)18)20-8-6-16(24)13-19(20)25/h5-8,11,13H,2-4,9-10,12H2,1H3,(H,27,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 363 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from CB1 receptor in CD-1 mouse cerebellum membrane after 1 hr by liquid scintillation counting method |
Bioorg Med Chem 24: 5291-5301 (2016)
Article DOI: 10.1016/j.bmc.2016.08.055
BindingDB Entry DOI: 10.7270/Q2HT2SV1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM22988

((5Z,8Z,11Z,14Z)-N-(2-hydroxyethyl)icosa-5,8,11,14-...)Show InChI InChI=1S/C22H37NO2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-22(25)23-20-21-24/h6-7,9-10,12-13,15-16,24H,2-5,8,11,14,17-21H2,1H3,(H,23,25)/b7-6-,10-9-,13-12-,16-15- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 371 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor (unknown origin) |
Bioorg Med Chem 24: 5291-5301 (2016)
Article DOI: 10.1016/j.bmc.2016.08.055
BindingDB Entry DOI: 10.7270/Q2HT2SV1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50180022

(5-(4-chloro-3-methyl-phenyl)-1-(4-methyl-benzyl)-1...)Show SMILES Cc1ccc(Cn2nc(cc2-c2ccc(Cl)c(C)c2)C(=O)NC2C3(C)CCC(C3)C2(C)C)cc1 |THB:21:22:26.25:28| Show InChI InChI=1S/C29H34ClN3O/c1-18-6-8-20(9-7-18)17-33-25(21-10-11-23(30)19(2)14-21)15-24(32-33)26(34)31-27-28(3,4)22-12-13-29(27,5)16-22/h6-11,14-15,22,27H,12-13,16-17H2,1-5H3,(H,31,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from rat brain CB1 receptor after 1 hr by scintillation counting method |
Bioorg Med Chem 24: 5291-5301 (2016)
Article DOI: 10.1016/j.bmc.2016.08.055
BindingDB Entry DOI: 10.7270/Q2HT2SV1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50180022

(5-(4-chloro-3-methyl-phenyl)-1-(4-methyl-benzyl)-1...)Show SMILES Cc1ccc(Cn2nc(cc2-c2ccc(Cl)c(C)c2)C(=O)NC2C3(C)CCC(C3)C2(C)C)cc1 |THB:21:22:26.25:28| Show InChI InChI=1S/C29H34ClN3O/c1-18-6-8-20(9-7-18)17-33-25(21-10-11-23(30)19(2)14-21)15-24(32-33)26(34)31-27-28(3,4)22-12-13-29(27,5)16-22/h6-11,14-15,22,27H,12-13,16-17H2,1-5H3,(H,31,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from human U937 cell-derived CB1 receptor expressed in CHO cell membrane after 1 hr by scintillation counting method |
Bioorg Med Chem 24: 5291-5301 (2016)
Article DOI: 10.1016/j.bmc.2016.08.055
BindingDB Entry DOI: 10.7270/Q2HT2SV1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM26144
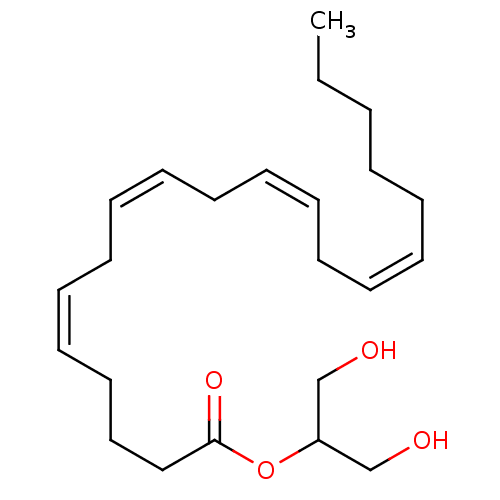
(1,3-dihydroxypropan-2-yl (5Z,8Z,11Z,14Z)-icosa-5,8...)Show SMILES CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)OC(CO)CO Show InChI InChI=1S/C23H38O4/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-23(26)27-22(20-24)21-25/h6-7,9-10,12-13,15-16,22,24-25H,2-5,8,11,14,17-21H2,1H3/b7-6-,10-9-,13-12-,16-15- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 472 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Binding affinity to CB1 receptor (unknown origin) |
Bioorg Med Chem 24: 5291-5301 (2016)
Article DOI: 10.1016/j.bmc.2016.08.055
BindingDB Entry DOI: 10.7270/Q2HT2SV1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50534083
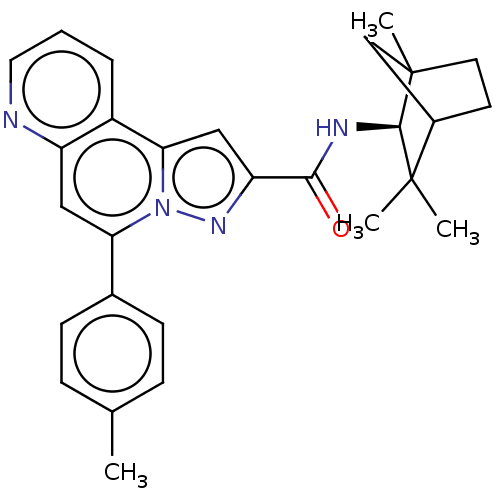
(CHEMBL4443516)Show SMILES Cc1ccc(cc1)-c1cc2ncccc2c2cc(nn12)C(=O)N[C@H]1C2(C)CCC(C2)C1(C)C |r,TLB:22:23:29:27.26| Show InChI InChI=1S/C28H30N4O/c1-17-7-9-18(10-8-17)23-14-21-20(6-5-13-29-21)24-15-22(31-32(23)24)25(33)30-26-27(2,3)19-11-12-28(26,4)16-19/h5-10,13-15,19,26H,11-12,16H2,1-4H3,(H,30,33)/t19?,26-,28?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from human CB2 receptor expressed in CHO cell membrane after 60 mins by scintillation counting method |
Bioorg Med Chem 24: 5291-5301 (2016)
Article DOI: 10.1016/j.bmc.2016.08.055
BindingDB Entry DOI: 10.7270/Q2HT2SV1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50534084

(CHEMBL4467772)Show SMILES Cc1ccc(cc1)-c1cc2ncccc2c2cc(nn12)C(=O)NN1CCCCC1 Show InChI InChI=1S/C23H23N5O/c1-16-7-9-17(10-8-16)21-14-19-18(6-5-11-24-19)22-15-20(25-28(21)22)23(29)26-27-12-3-2-4-13-27/h5-11,14-15H,2-4,12-13H2,1H3,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from human CB2 receptor expressed in CHO cell membrane after 60 mins by scintillation counting method |
Bioorg Med Chem 24: 5291-5301 (2016)
Article DOI: 10.1016/j.bmc.2016.08.055
BindingDB Entry DOI: 10.7270/Q2HT2SV1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM26144

(1,3-dihydroxypropan-2-yl (5Z,8Z,11Z,14Z)-icosa-5,8...)Show SMILES CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)OC(CO)CO Show InChI InChI=1S/C23H38O4/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-23(26)27-22(20-24)21-25/h6-7,9-10,12-13,15-16,22,24-25H,2-5,8,11,14,17-21H2,1H3/b7-6-,10-9-,13-12-,16-15- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor (unknown origin) |
Bioorg Med Chem 24: 5291-5301 (2016)
Article DOI: 10.1016/j.bmc.2016.08.055
BindingDB Entry DOI: 10.7270/Q2HT2SV1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50534086

(CHEMBL4461183)Show SMILES Cc1ccc(cc1)-c1cc2ncccc2c2cc(nn12)C(=O)NC1CCCCC1 Show InChI InChI=1S/C24H24N4O/c1-16-9-11-17(12-10-16)22-14-20-19(8-5-13-25-20)23-15-21(27-28(22)23)24(29)26-18-6-3-2-4-7-18/h5,8-15,18H,2-4,6-7H2,1H3,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from human CB2 receptor expressed in CHO cell membrane after 60 mins by scintillation counting method |
Bioorg Med Chem 24: 5291-5301 (2016)
Article DOI: 10.1016/j.bmc.2016.08.055
BindingDB Entry DOI: 10.7270/Q2HT2SV1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50534083

(CHEMBL4443516)Show SMILES Cc1ccc(cc1)-c1cc2ncccc2c2cc(nn12)C(=O)N[C@H]1C2(C)CCC(C2)C1(C)C |r,TLB:22:23:29:27.26| Show InChI InChI=1S/C28H30N4O/c1-17-7-9-18(10-8-17)23-14-21-20(6-5-13-29-21)24-15-22(31-32(23)24)25(33)30-26-27(2,3)19-11-12-28(26,4)16-19/h5-10,13-15,19,26H,11-12,16H2,1-4H3,(H,30,33)/t19?,26-,28?/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from human CB1 receptor expressed in CHO cell membrane after 90 mins by scintillation counting method |
Bioorg Med Chem 24: 5291-5301 (2016)
Article DOI: 10.1016/j.bmc.2016.08.055
BindingDB Entry DOI: 10.7270/Q2HT2SV1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50534087

(CHEMBL4520356)Show SMILES CC(C)[C@@H]1CC[C@@H](C)C[C@H]1NC(=O)c1cc2c3cccnc3cc(-c3ccc(C)cc3)n2n1 |r| Show InChI InChI=1S/C28H32N4O/c1-17(2)21-12-9-19(4)14-24(21)30-28(33)25-16-27-22-6-5-13-29-23(22)15-26(32(27)31-25)20-10-7-18(3)8-11-20/h5-8,10-11,13,15-17,19,21,24H,9,12,14H2,1-4H3,(H,30,33)/t19-,21+,24-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from human CB1 receptor expressed in CHO cell membrane after 90 mins by scintillation counting method |
Bioorg Med Chem 24: 5291-5301 (2016)
Article DOI: 10.1016/j.bmc.2016.08.055
BindingDB Entry DOI: 10.7270/Q2HT2SV1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50534085

(CHEMBL4458732)Show SMILES Cc1ccc(cc1)-c1cc2ncccc2c2cc(nn12)C(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:26:27:24.25.30:31,THB:26:25:31:32.27.28,28:27:24:30.29.31,28:29:24:32.26.27| Show InChI InChI=1S/C28H28N4O/c1-17-4-6-21(7-5-17)25-12-23-22(3-2-8-29-23)26-13-24(31-32(25)26)27(33)30-28-14-18-9-19(15-28)11-20(10-18)16-28/h2-8,12-13,18-20H,9-11,14-16H2,1H3,(H,30,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from human CB1 receptor expressed in CHO cell membrane after 90 mins by scintillation counting method |
Bioorg Med Chem 24: 5291-5301 (2016)
Article DOI: 10.1016/j.bmc.2016.08.055
BindingDB Entry DOI: 10.7270/Q2HT2SV1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50534088

(CHEMBL4453164)Show SMILES Cc1ccc(cc1)-c1cc2ncccc2c2cc(nn12)C(=O)N[C@H]1CC2CCC1(C)C2(C)C |r,TLB:22:23:27.26:30| Show InChI InChI=1S/C28H30N4O/c1-17-7-9-18(10-8-17)23-15-21-20(6-5-13-29-21)24-16-22(31-32(23)24)26(33)30-25-14-19-11-12-28(25,4)27(19,2)3/h5-10,13,15-16,19,25H,11-12,14H2,1-4H3,(H,30,33)/t19?,25-,28?/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from human CB1 receptor expressed in CHO cell membrane after 90 mins by scintillation counting method |
Bioorg Med Chem 24: 5291-5301 (2016)
Article DOI: 10.1016/j.bmc.2016.08.055
BindingDB Entry DOI: 10.7270/Q2HT2SV1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50534086

(CHEMBL4461183)Show SMILES Cc1ccc(cc1)-c1cc2ncccc2c2cc(nn12)C(=O)NC1CCCCC1 Show InChI InChI=1S/C24H24N4O/c1-16-9-11-17(12-10-16)22-14-20-19(8-5-13-25-20)23-15-21(27-28(22)23)24(29)26-18-6-3-2-4-7-18/h5,8-15,18H,2-4,6-7H2,1H3,(H,26,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from human CB1 receptor expressed in CHO cell membrane after 90 mins by scintillation counting method |
Bioorg Med Chem 24: 5291-5301 (2016)
Article DOI: 10.1016/j.bmc.2016.08.055
BindingDB Entry DOI: 10.7270/Q2HT2SV1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50534082

(CHEMBL4448088)Show SMILES CC1(C)C2CC1[C@H](CNC(=O)c1cc3c4cccnc4cc(-c4ccc(Cl)cc4Cl)n3n1)CC2 |r,TLB:7:6:1:4| Show InChI InChI=1S/C27H26Cl2N4O/c1-27(2)16-6-5-15(20(27)10-16)14-31-26(34)23-13-25-19-4-3-9-30-22(19)12-24(33(25)32-23)18-8-7-17(28)11-21(18)29/h3-4,7-9,11-13,15-16,20H,5-6,10,14H2,1-2H3,(H,31,34)/t15-,16?,20?/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from human CB1 receptor expressed in CHO cell membrane after 90 mins by scintillation counting method |
Bioorg Med Chem 24: 5291-5301 (2016)
Article DOI: 10.1016/j.bmc.2016.08.055
BindingDB Entry DOI: 10.7270/Q2HT2SV1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50534090

(CHEMBL4528488)Show SMILES Cc1ccc(cc1)-c1cc2ncccc2c2cc(nn12)C(=O)NC1C2CC3CC(C2)CC1C3 |TLB:22:23:25:29.28.27,THB:23:24:27:32.31.30,23:31:25.24.29:27,30:31:25:29.28.27,30:28:25:32.23.31,(14.71,-21.04,;13.39,-20.25,;12.05,-21,;10.72,-20.21,;10.74,-18.67,;12.1,-17.92,;13.41,-18.71,;9.42,-17.89,;8.08,-18.64,;6.75,-17.85,;5.41,-18.6,;4.09,-17.81,;4.11,-16.27,;5.45,-15.52,;6.78,-16.31,;8.12,-15.56,;8.46,-14.06,;10,-13.92,;10.6,-15.33,;9.44,-16.35,;10.78,-12.6,;10.03,-11.24,;12.31,-12.6,;12.7,-11.11,;14.1,-10.54,;15.6,-10.95,;14.4,-9.68,;14.4,-8.2,;13.05,-7.73,;14.1,-8.96,;11.66,-8.31,;11.68,-9.84,;13.07,-10.18,)| Show InChI InChI=1S/C28H28N4O/c1-16-4-6-19(7-5-16)25-14-23-22(3-2-8-29-23)26-15-24(31-32(25)26)28(33)30-27-20-10-17-9-18(12-20)13-21(27)11-17/h2-8,14-15,17-18,20-21,27H,9-13H2,1H3,(H,30,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from human CB1 receptor expressed in CHO cell membrane after 90 mins by scintillation counting method |
Bioorg Med Chem 24: 5291-5301 (2016)
Article DOI: 10.1016/j.bmc.2016.08.055
BindingDB Entry DOI: 10.7270/Q2HT2SV1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50534084

(CHEMBL4467772)Show SMILES Cc1ccc(cc1)-c1cc2ncccc2c2cc(nn12)C(=O)NN1CCCCC1 Show InChI InChI=1S/C23H23N5O/c1-16-7-9-17(10-8-16)21-14-19-18(6-5-11-24-19)22-15-20(25-28(21)22)23(29)26-27-12-3-2-4-13-27/h5-11,14-15H,2-4,12-13H2,1H3,(H,26,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from human CB1 receptor expressed in CHO cell membrane after 90 mins by scintillation counting method |
Bioorg Med Chem 24: 5291-5301 (2016)
Article DOI: 10.1016/j.bmc.2016.08.055
BindingDB Entry DOI: 10.7270/Q2HT2SV1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50534091

(CHEMBL4525460)Show SMILES Cc1ccc(cc1)-c1cc2ncccc2c2cc(nn12)C(=O)NC[C@@H]1CCC2CC1C2(C)C |r,THB:23:24:30:28| Show InChI InChI=1S/C28H30N4O/c1-17-6-8-18(9-7-17)25-14-23-21(5-4-12-29-23)26-15-24(31-32(25)26)27(33)30-16-19-10-11-20-13-22(19)28(20,2)3/h4-9,12,14-15,19-20,22H,10-11,13,16H2,1-3H3,(H,30,33)/t19-,20?,22?/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from human CB1 receptor expressed in CHO cell membrane after 90 mins by scintillation counting method |
Bioorg Med Chem 24: 5291-5301 (2016)
Article DOI: 10.1016/j.bmc.2016.08.055
BindingDB Entry DOI: 10.7270/Q2HT2SV1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50534089

(CHEMBL4549921)Show SMILES C[C@H]1CC2CC(C1NC(=O)c1cc3c4cccnc4cc(-c4ccc(C)cc4)n3n1)C2(C)C |r,TLB:7:6:4:30,THB:0:1:4:30| Show InChI InChI=1S/C28H30N4O/c1-16-7-9-18(10-8-16)24-14-22-20(6-5-11-29-22)25-15-23(31-32(24)25)27(33)30-26-17(2)12-19-13-21(26)28(19,3)4/h5-11,14-15,17,19,21,26H,12-13H2,1-4H3,(H,30,33)/t17-,19?,21?,26?/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from human CB1 receptor expressed in CHO cell membrane after 90 mins by scintillation counting method |
Bioorg Med Chem 24: 5291-5301 (2016)
Article DOI: 10.1016/j.bmc.2016.08.055
BindingDB Entry DOI: 10.7270/Q2HT2SV1 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM31596
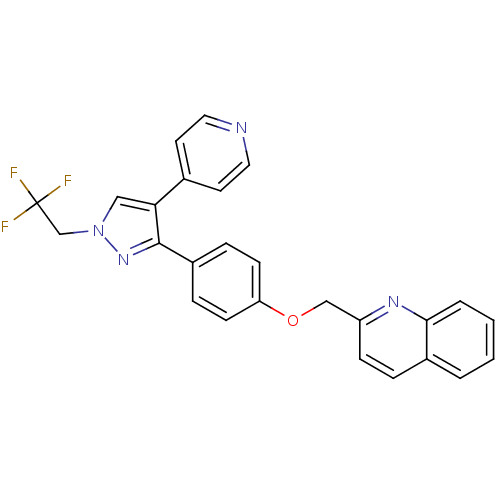
(substituted pyrazole, 13)Show SMILES FC(F)(F)Cn1cc(c(n1)-c1ccc(OCc2ccc3ccccc3n2)cc1)-c1ccncc1 Show InChI InChI=1S/C26H19F3N4O/c27-26(28,29)17-33-15-23(18-11-13-30-14-12-18)25(32-33)20-6-9-22(10-7-20)34-16-21-8-5-19-3-1-2-4-24(19)31-21/h1-15H,16-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of human PDE10A (amino acids 14 to 779) using [3H]-labelled cyclic nucleotide as substrate after 1 hr b beta counting |
Eur J Med Chem 84: 181-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.020
BindingDB Entry DOI: 10.7270/Q2S1844K |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50049925

(CHEMBL3317682)Show SMILES Cc1cc2c3cccnc3cc(CCc3nc(cn3C)-c3ccccc3)n2n1 Show InChI InChI=1S/C23H21N5/c1-16-13-22-19-9-6-12-24-20(19)14-18(28(22)26-16)10-11-23-25-21(15-27(23)2)17-7-4-3-5-8-17/h3-9,12-15H,10-11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of human PDE10A (amino acids 14 to 779) using [3H]-labelled cyclic nucleotide as substrate after 1 hr b beta counting |
Eur J Med Chem 84: 181-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.020
BindingDB Entry DOI: 10.7270/Q2S1844K |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50049931
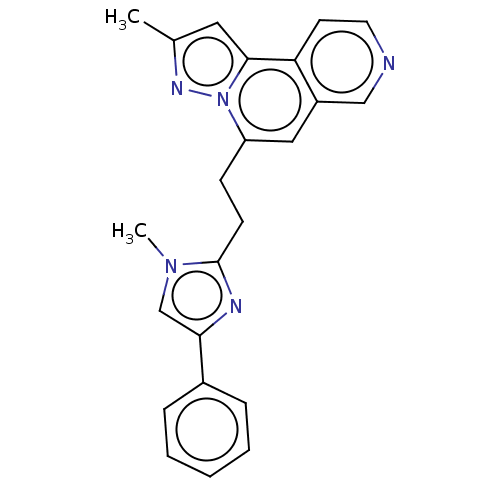
(CHEMBL3317688)Show SMILES Cc1cc2c3ccncc3cc(CCc3nc(cn3C)-c3ccccc3)n2n1 Show InChI InChI=1S/C23H21N5/c1-16-12-22-20-10-11-24-14-18(20)13-19(28(22)26-16)8-9-23-25-21(15-27(23)2)17-6-4-3-5-7-17/h3-7,10-15H,8-9H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of human PDE10A (amino acids 14 to 779) using [3H]-labelled cyclic nucleotide as substrate after 1 hr b beta counting |
Eur J Med Chem 84: 181-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.020
BindingDB Entry DOI: 10.7270/Q2S1844K |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50049930

(CHEMBL3317687)Show SMILES COCc1cc2c3cccnc3cc(CCc3nc(cn3C)-c3ccccc3)n2n1 Show InChI InChI=1S/C24H23N5O/c1-28-15-22(17-7-4-3-5-8-17)26-24(28)11-10-19-14-21-20(9-6-12-25-21)23-13-18(16-30-2)27-29(19)23/h3-9,12-15H,10-11,16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of human PDE10A (amino acids 14 to 779) using [3H]-labelled cyclic nucleotide as substrate after 1 hr b beta counting |
Eur J Med Chem 84: 181-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.020
BindingDB Entry DOI: 10.7270/Q2S1844K |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50334662
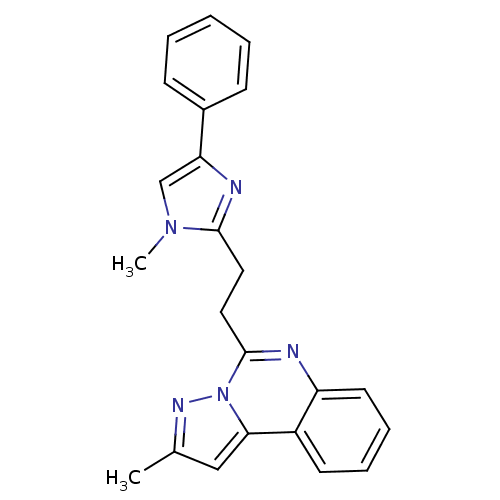
(2-Methyl-5-(2-(1-methyl-4-phenyl-1H-imidazol-2-yl)...)Show SMILES Cc1cc2c3ccccc3nc(CCc3nc(cn3C)-c3ccccc3)n2n1 Show InChI InChI=1S/C23H21N5/c1-16-14-21-18-10-6-7-11-19(18)24-23(28(21)26-16)13-12-22-25-20(15-27(22)2)17-8-4-3-5-9-17/h3-11,14-15H,12-13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of human PDE10A (amino acids 14 to 779) using [3H]-labelled cyclic nucleotide as substrate after 1 hr b beta counting |
Eur J Med Chem 84: 181-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.020
BindingDB Entry DOI: 10.7270/Q2S1844K |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50049932

(CHEMBL3317689)Show SMILES Cc1cc2c3cnccc3cc(CCc3nc(cn3C)-c3ccccc3)n2n1 Show InChI InChI=1S/C23H21N5/c1-16-12-22-20-14-24-11-10-18(20)13-19(28(22)26-16)8-9-23-25-21(15-27(23)2)17-6-4-3-5-7-17/h3-7,10-15H,8-9H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of human PDE10A (amino acids 14 to 779) using [3H]-labelled cyclic nucleotide as substrate after 1 hr b beta counting |
Eur J Med Chem 84: 181-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.020
BindingDB Entry DOI: 10.7270/Q2S1844K |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50049926

(CHEMBL3317683)Show InChI InChI=1S/C21H19N5/c1-14-12-20-16-6-5-11-22-18(16)13-15(26(20)24-14)9-10-21-23-17-7-3-4-8-19(17)25(21)2/h3-8,11-13H,9-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of human PDE10A (amino acids 14 to 779) using [3H]-labelled cyclic nucleotide as substrate after 1 hr b beta counting |
Eur J Med Chem 84: 181-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.020
BindingDB Entry DOI: 10.7270/Q2S1844K |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50049929
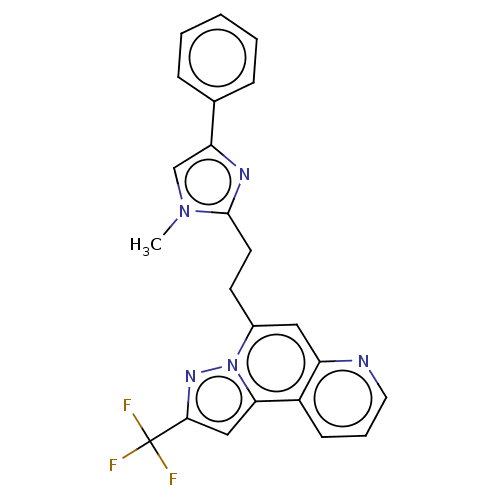
(CHEMBL3317686)Show SMILES Cn1cc(nc1CCc1cc2ncccc2c2cc(nn12)C(F)(F)F)-c1ccccc1 Show InChI InChI=1S/C23H18F3N5/c1-30-14-19(15-6-3-2-4-7-15)28-22(30)10-9-16-12-18-17(8-5-11-27-18)20-13-21(23(24,25)26)29-31(16)20/h2-8,11-14H,9-10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of human PDE10A (amino acids 14 to 779) using [3H]-labelled cyclic nucleotide as substrate after 1 hr b beta counting |
Eur J Med Chem 84: 181-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.020
BindingDB Entry DOI: 10.7270/Q2S1844K |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50049927

(CHEMBL3317684)Show SMILES CCOC(=O)c1cc2c3cccnc3cc(CCc3nc(cn3C)-c3ccccc3)n2n1 Show InChI InChI=1S/C25H23N5O2/c1-3-32-25(31)21-15-23-19-10-7-13-26-20(19)14-18(30(23)28-21)11-12-24-27-22(16-29(24)2)17-8-5-4-6-9-17/h4-10,13-16H,3,11-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of human PDE10A (amino acids 14 to 779) using [3H]-labelled cyclic nucleotide as substrate after 1 hr b beta counting |
Eur J Med Chem 84: 181-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.020
BindingDB Entry DOI: 10.7270/Q2S1844K |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50049933

(CHEMBL3317690)Show InChI InChI=1S/C21H19N5/c1-14-11-20-17-13-22-10-9-15(17)12-16(26(20)24-14)7-8-21-23-18-5-3-4-6-19(18)25(21)2/h3-6,9-13H,7-8H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of human PDE10A (amino acids 14 to 779) using [3H]-labelled cyclic nucleotide as substrate after 1 hr b beta counting |
Eur J Med Chem 84: 181-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.020
BindingDB Entry DOI: 10.7270/Q2S1844K |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50049934

(CHEMBL3317691)Show SMILES Cc1cc2c3ccccc3cc(CCc3nc(cn3C)-c3ccccc3)n2n1 Show InChI InChI=1S/C24H22N4/c1-17-14-23-21-11-7-6-10-19(21)15-20(28(23)26-17)12-13-24-25-22(16-27(24)2)18-8-4-3-5-9-18/h3-11,14-16H,12-13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of human PDE10A (amino acids 14 to 779) using [3H]-labelled cyclic nucleotide as substrate after 1 hr b beta counting |
Eur J Med Chem 84: 181-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.020
BindingDB Entry DOI: 10.7270/Q2S1844K |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50049935

(CHEMBL3317692)Show InChI InChI=1S/C22H20N4/c1-15-13-21-18-8-4-3-7-16(18)14-17(26(21)24-15)11-12-22-23-19-9-5-6-10-20(19)25(22)2/h3-10,13-14H,11-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of human PDE10A (amino acids 14 to 779) using [3H]-labelled cyclic nucleotide as substrate after 1 hr b beta counting |
Eur J Med Chem 84: 181-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.020
BindingDB Entry DOI: 10.7270/Q2S1844K |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50049928

(CHEMBL3317685)Show SMILES CCOC(=O)c1cc2c3cccnc3cc(CCc3nc4ccccc4n3C)n2n1 Show InChI InChI=1S/C23H21N5O2/c1-3-30-23(29)19-14-21-16-7-6-12-24-18(16)13-15(28(21)26-19)10-11-22-25-17-8-4-5-9-20(17)27(22)2/h4-9,12-14H,3,10-11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of human PDE10A (amino acids 14 to 779) using [3H]-labelled cyclic nucleotide as substrate after 1 hr b beta counting |
Eur J Med Chem 84: 181-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.020
BindingDB Entry DOI: 10.7270/Q2S1844K |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data