

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM20461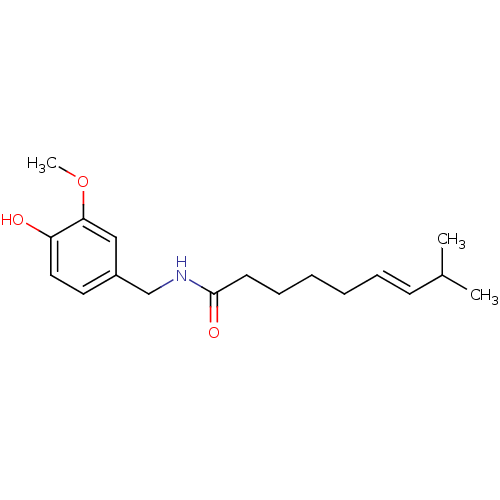 ((6E)-N-[(4-hydroxy-3-methoxyphenyl)methyl]-8-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Antagonist activity against human recombinant TRPV1 expressed in HEK293 cells assessed as inhibition of capsaicin-induced Ca2+ influx pre-treated 5 m... | Bioorg Med Chem Lett 25: 1009-11 (2015) Article DOI: 10.1016/j.bmcl.2015.01.039 BindingDB Entry DOI: 10.7270/Q2VD717Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM22999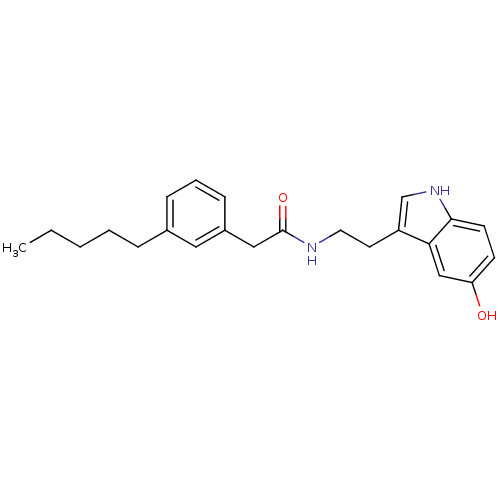 (N-[2-(5-hydroxy-1H-indol-3-yl)ethyl]-2-(3-pentylph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rome La Sapienza | Assay Description The antagonistic effects of test compounds were evaluated using HEK293 cells stably overexpressing human TRPV1. Experiments were carried out by measu... | J Med Chem 50: 6554-69 (2007) Article DOI: 10.1021/jm070678q BindingDB Entry DOI: 10.7270/Q2JH3JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM22987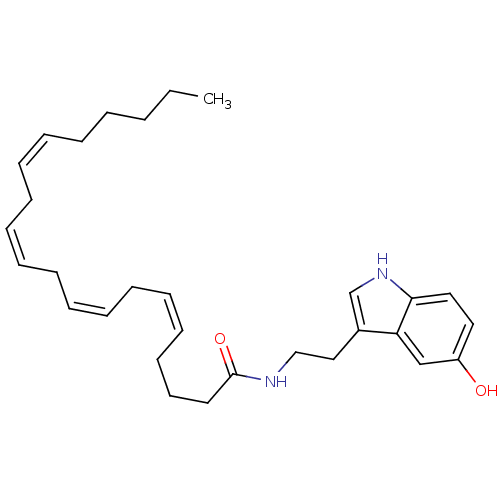 ((5Z,8Z,11Z,14Z)-N-[2-(5-hydroxy-1H-indol-3-yl)ethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rome La Sapienza | Assay Description The antagonistic effects of test compounds were evaluated using HEK293 cells stably overexpressing human TRPV1. Experiments were carried out by measu... | J Med Chem 50: 6554-69 (2007) Article DOI: 10.1021/jm070678q BindingDB Entry DOI: 10.7270/Q2JH3JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM23000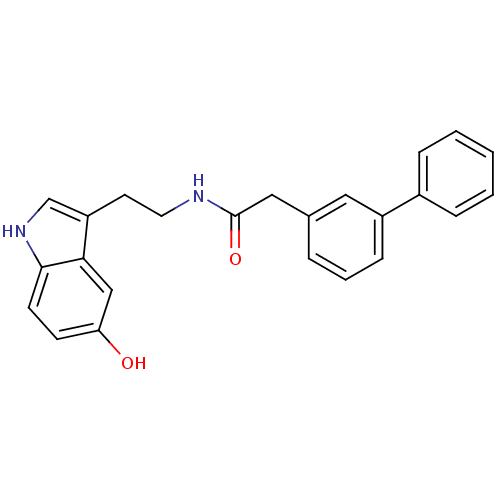 (JMC506554 Compound 1m | N-[2-(5-hydroxy-1H-indol-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rome La Sapienza | Assay Description The antagonistic effects of test compounds were evaluated using HEK293 cells stably overexpressing human TRPV1. Experiments were carried out by measu... | J Med Chem 50: 6554-69 (2007) Article DOI: 10.1021/jm070678q BindingDB Entry DOI: 10.7270/Q2JH3JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Rattus norvegicus) | BDBM50318484 (2-((1R,6R)-6-Isopropenyl-3-methyl-cyclohex-2-enyl)...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at rat TRPA1 expressed in HEK293 cells assessed as inhibition of allylisothiocyanate-induced Ca2+ response preincubated for 5 min... | Citation and Details Article DOI: 10.1021/acs.jnatprod.0c00668 BindingDB Entry DOI: 10.7270/Q2ZW1QJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM22989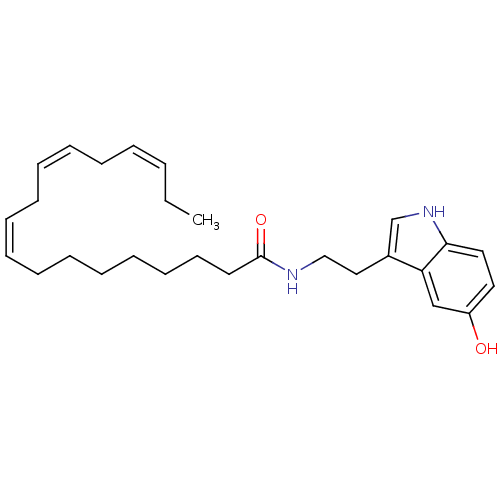 ((9Z,12Z,15Z)-N-[2-(5-hydroxy-1H-indol-3-yl)ethyl]o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rome La Sapienza | Assay Description The antagonistic effects of test compounds were evaluated using HEK293 cells stably overexpressing human TRPV1. Experiments were carried out by measu... | J Med Chem 50: 6554-69 (2007) Article DOI: 10.1021/jm070678q BindingDB Entry DOI: 10.7270/Q2JH3JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM23015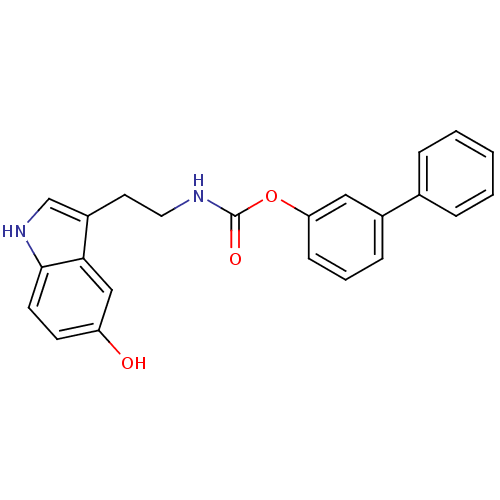 (3-phenylphenyl N-[2-(5-hydroxy-1H-indol-3-yl)ethyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | 9.0 | 37 |
University of Rome La Sapienza | Assay Description The effect of the test compounds on the enzymatic hydrolysis of [14C]anandamide was evaluated by using membranes prepared from rat brain. [14C]Ethano... | J Med Chem 50: 6554-69 (2007) Article DOI: 10.1021/jm070678q BindingDB Entry DOI: 10.7270/Q2JH3JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM22998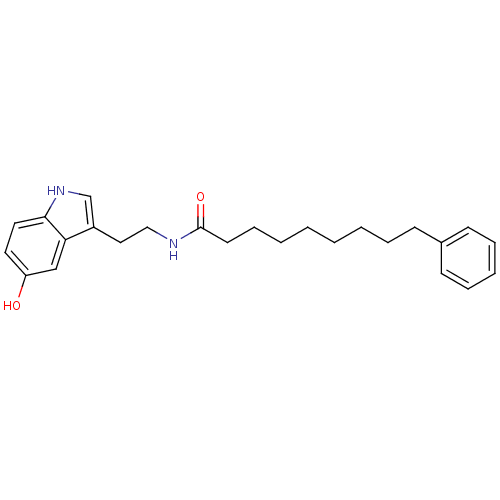 (N-[2-(5-hydroxy-1H-indol-3-yl)ethyl]-9-phenylnonan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rome La Sapienza | Assay Description The antagonistic effects of test compounds were evaluated using HEK293 cells stably overexpressing human TRPV1. Experiments were carried out by measu... | J Med Chem 50: 6554-69 (2007) Article DOI: 10.1021/jm070678q BindingDB Entry DOI: 10.7270/Q2JH3JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM23007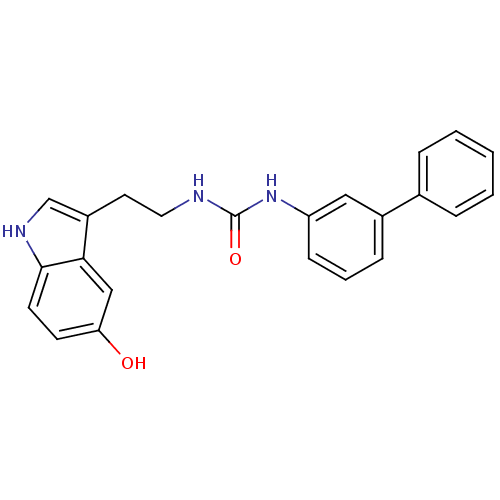 (3-[2-(5-hydroxy-1H-indol-3-yl)ethyl]-1-(3-phenylph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rome La Sapienza | Assay Description The antagonistic effects of test compounds were evaluated using HEK293 cells stably overexpressing human TRPV1. Experiments were carried out by measu... | J Med Chem 50: 6554-69 (2007) Article DOI: 10.1021/jm070678q BindingDB Entry DOI: 10.7270/Q2JH3JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM22995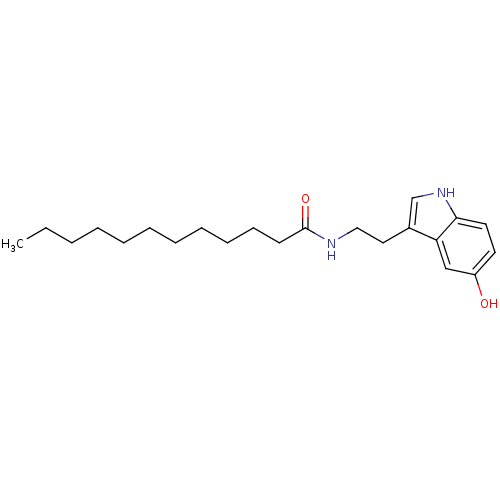 (N-[2-(5-hydroxy-1H-indol-3-yl)ethyl]dodecanamide |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rome La Sapienza | Assay Description The antagonistic effects of test compounds were evaluated using HEK293 cells stably overexpressing human TRPV1. Experiments were carried out by measu... | J Med Chem 50: 6554-69 (2007) Article DOI: 10.1021/jm070678q BindingDB Entry DOI: 10.7270/Q2JH3JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat sensitive channel TRPV3 (Rattus norvegicus) | BDBM50318484 (2-((1R,6R)-6-Isopropenyl-3-methyl-cyclohex-2-enyl)...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at rat TRPV3 expressed in HEK293 cells assessed as inhibition of thymol-induced Ca2+ response preincubated for 5 mins followed by... | Citation and Details Article DOI: 10.1021/acs.jnatprod.0c00668 BindingDB Entry DOI: 10.7270/Q2ZW1QJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM22994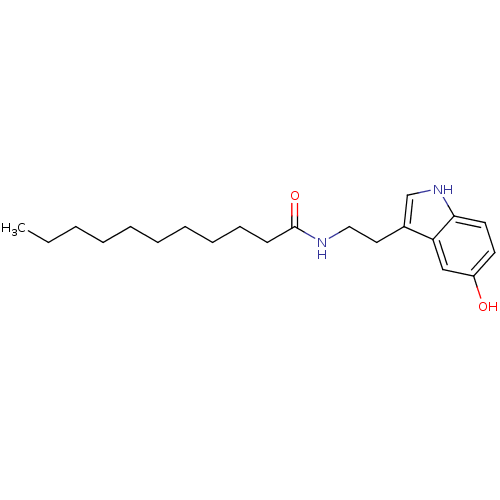 (N-[2-(5-hydroxy-1H-indol-3-yl)ethyl]undecanamide |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rome La Sapienza | Assay Description The antagonistic effects of test compounds were evaluated using HEK293 cells stably overexpressing human TRPV1. Experiments were carried out by measu... | J Med Chem 50: 6554-69 (2007) Article DOI: 10.1021/jm070678q BindingDB Entry DOI: 10.7270/Q2JH3JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM22990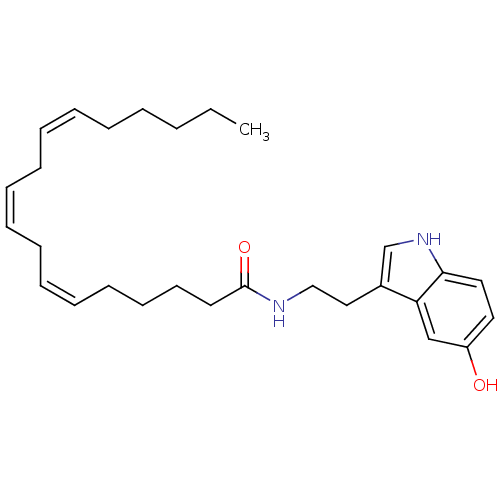 ((6Z,9Z,12Z)-N-[2-(5-hydroxy-1H-indol-3-yl)ethyl]oc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rome La Sapienza | Assay Description The antagonistic effects of test compounds were evaluated using HEK293 cells stably overexpressing human TRPV1. Experiments were carried out by measu... | J Med Chem 50: 6554-69 (2007) Article DOI: 10.1021/jm070678q BindingDB Entry DOI: 10.7270/Q2JH3JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Rattus norvegicus) | BDBM50103439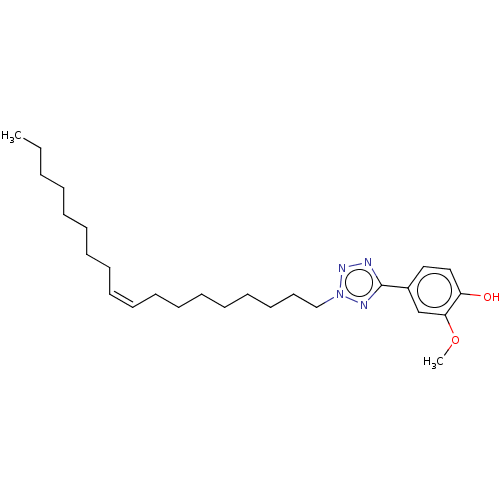 (CHEMBL3398239) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Antagonist activity against rat recombinant TRPA1 expressed in HEK293 cells assessed as inhibition of AITC-induced Ca2+ influx pre-treated 5 mins bef... | Bioorg Med Chem Lett 25: 1009-11 (2015) Article DOI: 10.1016/j.bmcl.2015.01.039 BindingDB Entry DOI: 10.7270/Q2VD717Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 2 (Rattus norvegicus) | BDBM50318484 (2-((1R,6R)-6-Isopropenyl-3-methyl-cyclohex-2-enyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at rat TRPV2 expressed in HEK293 cells assessed as inhibition of LPC-induced Ca2+ response preincubated for 5 mins followed by LP... | Citation and Details Article DOI: 10.1021/acs.jnatprod.0c00668 BindingDB Entry DOI: 10.7270/Q2ZW1QJH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM23026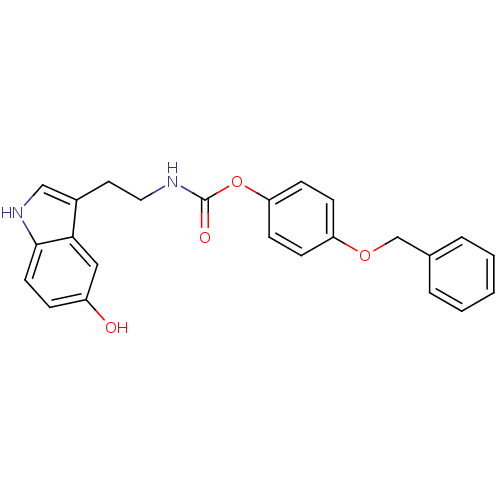 (4-(benzyloxy)phenyl N-[2-(5-hydroxy-1H-indol-3-yl)...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rome La Sapienza | Assay Description The effect of the test compounds on the enzymatic hydrolysis of [14C]anandamide was evaluated by using membranes prepared from rat brain. [14C]Ethano... | J Med Chem 50: 6554-69 (2007) Article DOI: 10.1021/jm070678q BindingDB Entry DOI: 10.7270/Q2JH3JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Rattus norvegicus) | BDBM50103440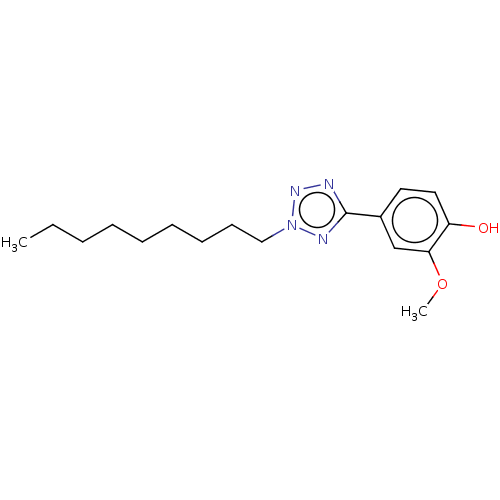 (CHEMBL3398238) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Antagonist activity against rat recombinant TRPA1 expressed in HEK293 cells assessed as inhibition of AITC-induced Ca2+ influx pre-treated 5 mins bef... | Bioorg Med Chem Lett 25: 1009-11 (2015) Article DOI: 10.1016/j.bmcl.2015.01.039 BindingDB Entry DOI: 10.7270/Q2VD717Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM23022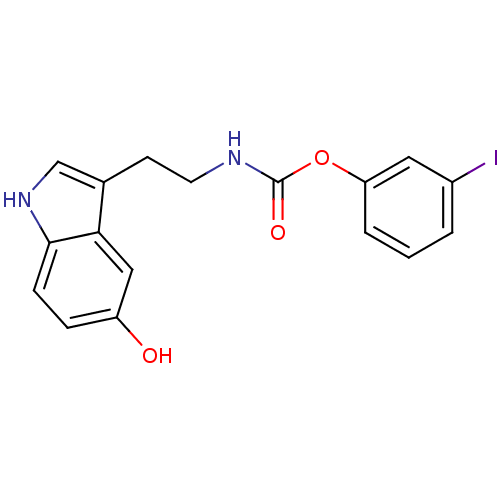 (3-iodophenyl N-[2-(5-hydroxy-1H-indol-3-yl)ethyl]c...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rome La Sapienza | Assay Description The effect of the test compounds on the enzymatic hydrolysis of [14C]anandamide was evaluated by using membranes prepared from rat brain. [14C]Ethano... | J Med Chem 50: 6554-69 (2007) Article DOI: 10.1021/jm070678q BindingDB Entry DOI: 10.7270/Q2JH3JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM22991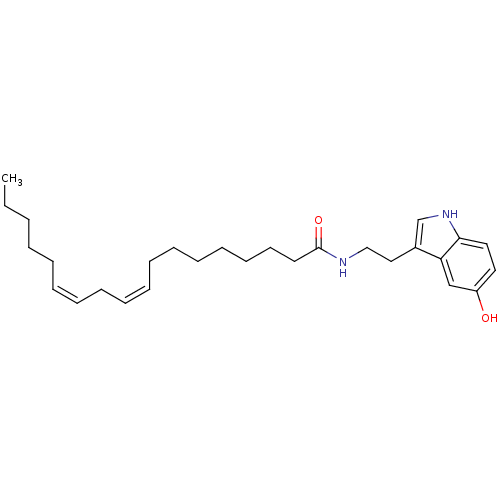 ((9Z,12Z)-N-[2-(5-hydroxy-1H-indol-3-yl)ethyl]octad...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rome La Sapienza | Assay Description The antagonistic effects of test compounds were evaluated using HEK293 cells stably overexpressing human TRPV1. Experiments were carried out by measu... | J Med Chem 50: 6554-69 (2007) Article DOI: 10.1021/jm070678q BindingDB Entry DOI: 10.7270/Q2JH3JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM23020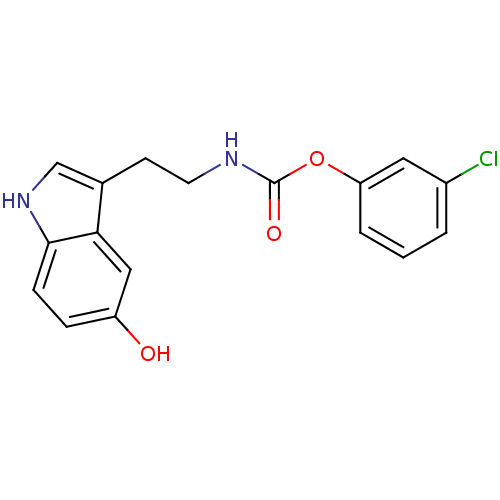 (3-chlorophenyl N-[2-(5-hydroxy-1H-indol-3-yl)ethyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rome La Sapienza | Assay Description The effect of the test compounds on the enzymatic hydrolysis of [14C]anandamide was evaluated by using membranes prepared from rat brain. [14C]Ethano... | J Med Chem 50: 6554-69 (2007) Article DOI: 10.1021/jm070678q BindingDB Entry DOI: 10.7270/Q2JH3JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM22997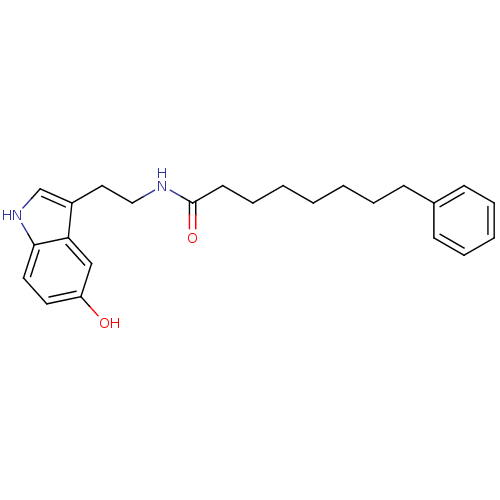 (N-[2-(5-hydroxy-1H-indol-3-yl)ethyl]-8-phenyloctan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rome La Sapienza | Assay Description The antagonistic effects of test compounds were evaluated using HEK293 cells stably overexpressing human TRPV1. Experiments were carried out by measu... | J Med Chem 50: 6554-69 (2007) Article DOI: 10.1021/jm070678q BindingDB Entry DOI: 10.7270/Q2JH3JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM23018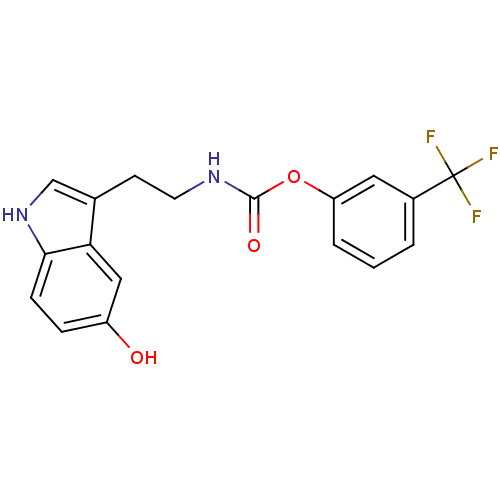 (3-(trifluoromethyl)phenyl N-[2-(5-hydroxy-1H-indol...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rome La Sapienza | Assay Description The effect of the test compounds on the enzymatic hydrolysis of [14C]anandamide was evaluated by using membranes prepared from rat brain. [14C]Ethano... | J Med Chem 50: 6554-69 (2007) Article DOI: 10.1021/jm070678q BindingDB Entry DOI: 10.7270/Q2JH3JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM23014 (3-pentylphenyl N-[2-(5-hydroxy-1H-indol-3-yl)ethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rome La Sapienza | Assay Description The antagonistic effects of test compounds were evaluated using HEK293 cells stably overexpressing human TRPV1. Experiments were carried out by measu... | J Med Chem 50: 6554-69 (2007) Article DOI: 10.1021/jm070678q BindingDB Entry DOI: 10.7270/Q2JH3JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM22992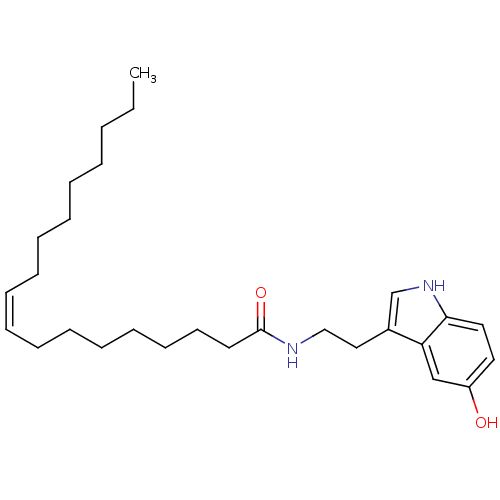 ((9Z)-N-[2-(5-hydroxy-1H-indol-3-yl)ethyl]octadec-9...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rome La Sapienza | Assay Description The antagonistic effects of test compounds were evaluated using HEK293 cells stably overexpressing human TRPV1. Experiments were carried out by measu... | J Med Chem 50: 6554-69 (2007) Article DOI: 10.1021/jm070678q BindingDB Entry DOI: 10.7270/Q2JH3JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM23004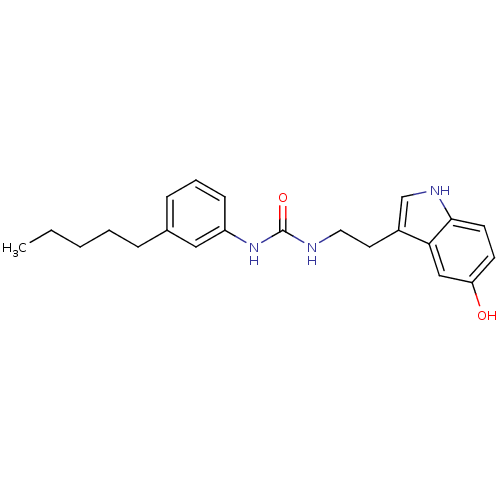 (3-[2-(5-hydroxy-1H-indol-3-yl)ethyl]-1-(3-pentylph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rome La Sapienza | Assay Description The antagonistic effects of test compounds were evaluated using HEK293 cells stably overexpressing human TRPV1. Experiments were carried out by measu... | J Med Chem 50: 6554-69 (2007) Article DOI: 10.1021/jm070678q BindingDB Entry DOI: 10.7270/Q2JH3JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily M member 8 (Rattus norvegicus (Rat)) | BDBM50318484 (2-((1R,6R)-6-Isopropenyl-3-methyl-cyclohex-2-enyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at rat TRPM8 expressed in HEK293 cells assessed as inhibition of icilin-induced Ca2+ response preincubated for 5 mins followed by... | Citation and Details Article DOI: 10.1021/acs.jnatprod.0c00668 BindingDB Entry DOI: 10.7270/Q2ZW1QJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM23010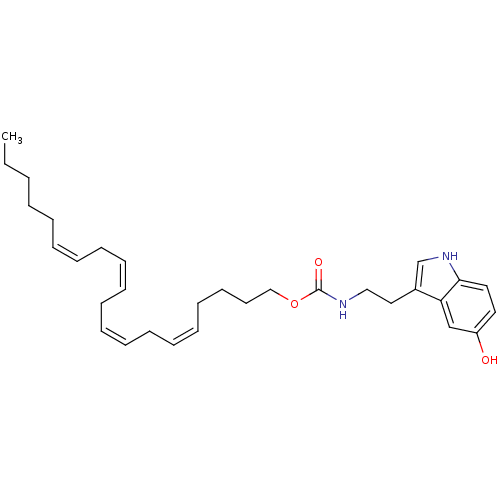 ((5Z,8Z,11Z,14Z)-icosa-5,8,11,14-tetraen-1-yl N-[2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rome La Sapienza | Assay Description The antagonistic effects of test compounds were evaluated using HEK293 cells stably overexpressing human TRPV1. Experiments were carried out by measu... | J Med Chem 50: 6554-69 (2007) Article DOI: 10.1021/jm070678q BindingDB Entry DOI: 10.7270/Q2JH3JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Rattus norvegicus) | BDBM50103441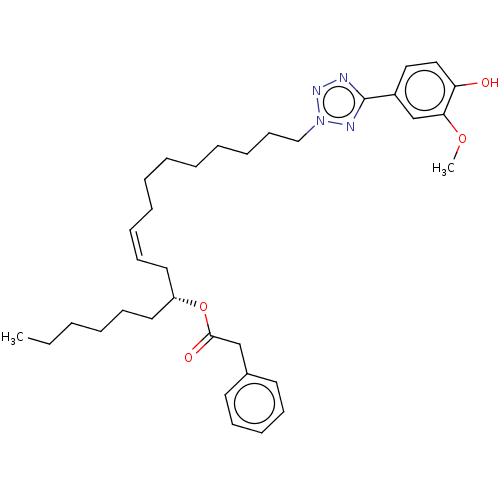 (CHEMBL3398241) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Antagonist activity against rat recombinant TRPA1 expressed in HEK293 cells assessed as inhibition of AITC-induced Ca2+ influx pre-treated 5 mins bef... | Bioorg Med Chem Lett 25: 1009-11 (2015) Article DOI: 10.1016/j.bmcl.2015.01.039 BindingDB Entry DOI: 10.7270/Q2VD717Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM23014 (3-pentylphenyl N-[2-(5-hydroxy-1H-indol-3-yl)ethyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | 9.0 | 37 |
University of Rome La Sapienza | Assay Description The effect of the test compounds on the enzymatic hydrolysis of [14C]anandamide was evaluated by using membranes prepared from rat brain. [14C]Ethano... | J Med Chem 50: 6554-69 (2007) Article DOI: 10.1021/jm070678q BindingDB Entry DOI: 10.7270/Q2JH3JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50318484 (2-((1R,6R)-6-Isopropenyl-3-methyl-cyclohex-2-enyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human TRPV1 expressed in HEK293 cells assessed as inhibition of capsaicin-induced Ca2+ response preincubated for 5 mins follow... | Citation and Details Article DOI: 10.1021/acs.jnatprod.0c00668 BindingDB Entry DOI: 10.7270/Q2ZW1QJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM23016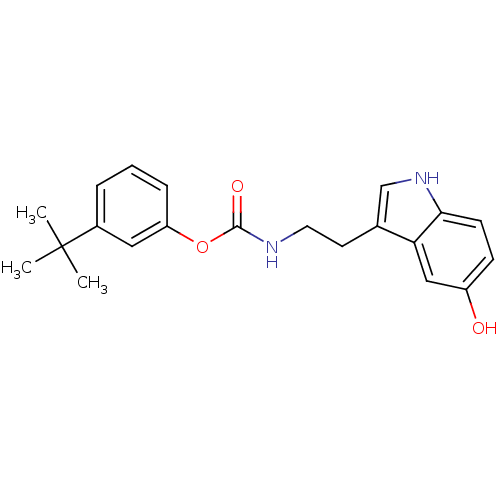 (3-tert-butylphenyl N-[2-(5-hydroxy-1H-indol-3-yl)e...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | 9.0 | 37 |
University of Rome La Sapienza | Assay Description The effect of the test compounds on the enzymatic hydrolysis of [14C]anandamide was evaluated by using membranes prepared from rat brain. [14C]Ethano... | J Med Chem 50: 6554-69 (2007) Article DOI: 10.1021/jm070678q BindingDB Entry DOI: 10.7270/Q2JH3JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM23003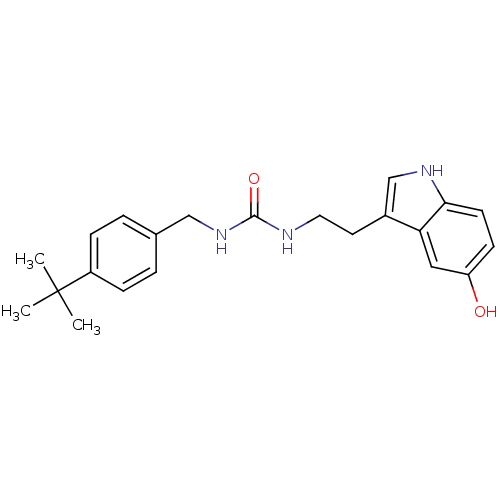 (1-[(4-tert-butylphenyl)methyl]-3-[2-(5-hydroxy-1H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rome La Sapienza | Assay Description The antagonistic effects of test compounds were evaluated using HEK293 cells stably overexpressing human TRPV1. Experiments were carried out by measu... | J Med Chem 50: 6554-69 (2007) Article DOI: 10.1021/jm070678q BindingDB Entry DOI: 10.7270/Q2JH3JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily M member 8 (Rattus norvegicus (Rat)) | BDBM50548384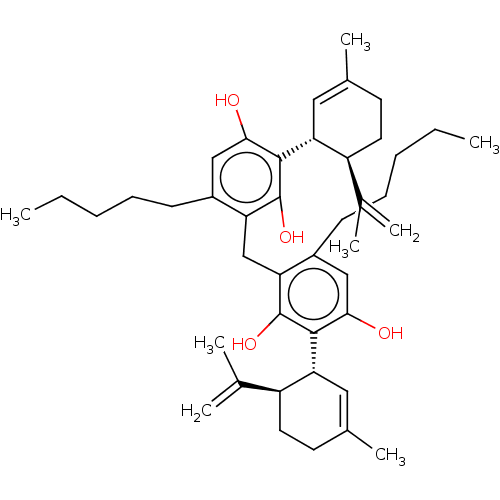 (CHEMBL4762439) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at rat TRPM8 expressed in HEK293 cells assessed as inhibition of icilin-induced Ca2+ response preincubated for 5 mins followed by... | Citation and Details Article DOI: 10.1021/acs.jnatprod.0c00668 BindingDB Entry DOI: 10.7270/Q2ZW1QJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Rattus norvegicus) | BDBM50548384 (CHEMBL4762439) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at rat TRPA1 expressed in HEK293 cells assessed as inhibition of allylisothiocyanate-induced Ca2+ response preincubated for 5 min... | Citation and Details Article DOI: 10.1021/acs.jnatprod.0c00668 BindingDB Entry DOI: 10.7270/Q2ZW1QJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM23023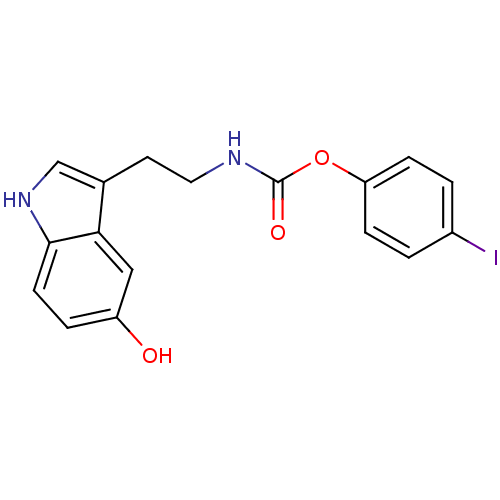 (4-iodophenyl N-[2-(5-hydroxy-1H-indol-3-yl)ethyl]c...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rome La Sapienza | Assay Description The effect of the test compounds on the enzymatic hydrolysis of [14C]anandamide was evaluated by using membranes prepared from rat brain. [14C]Ethano... | J Med Chem 50: 6554-69 (2007) Article DOI: 10.1021/jm070678q BindingDB Entry DOI: 10.7270/Q2JH3JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM23017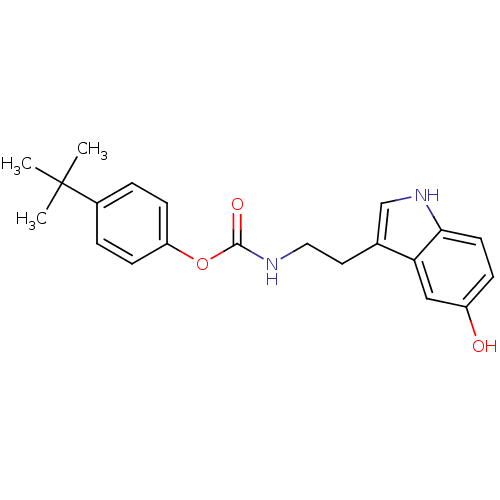 (4-tert-butylphenyl N-[2-(5-hydroxy-1H-indol-3-yl)e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rome La Sapienza | Assay Description The antagonistic effects of test compounds were evaluated using HEK293 cells stably overexpressing human TRPV1. Experiments were carried out by measu... | J Med Chem 50: 6554-69 (2007) Article DOI: 10.1021/jm070678q BindingDB Entry DOI: 10.7270/Q2JH3JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Rattus norvegicus) | BDBM50318484 (2-((1R,6R)-6-Isopropenyl-3-methyl-cyclohex-2-enyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at rat TRPV4 expressed in HEK293 cells assessed as inhibition of GSK1016790A-induced Ca2+ response preincubated for 5 mins follow... | Citation and Details Article DOI: 10.1021/acs.jnatprod.0c00668 BindingDB Entry DOI: 10.7270/Q2ZW1QJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM23019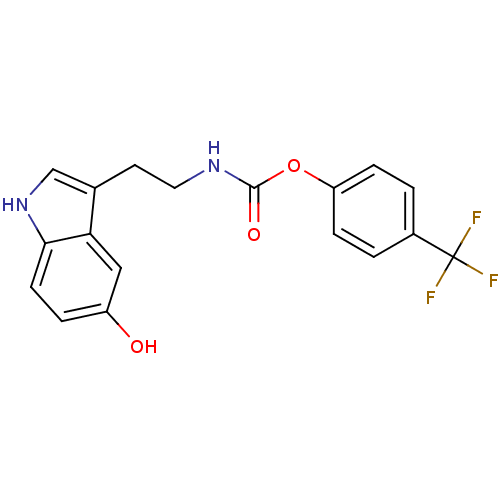 (4-(trifluoromethyl)phenyl N-[2-(5-hydroxy-1H-indol...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rome La Sapienza | Assay Description The effect of the test compounds on the enzymatic hydrolysis of [14C]anandamide was evaluated by using membranes prepared from rat brain. [14C]Ethano... | J Med Chem 50: 6554-69 (2007) Article DOI: 10.1021/jm070678q BindingDB Entry DOI: 10.7270/Q2JH3JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM23021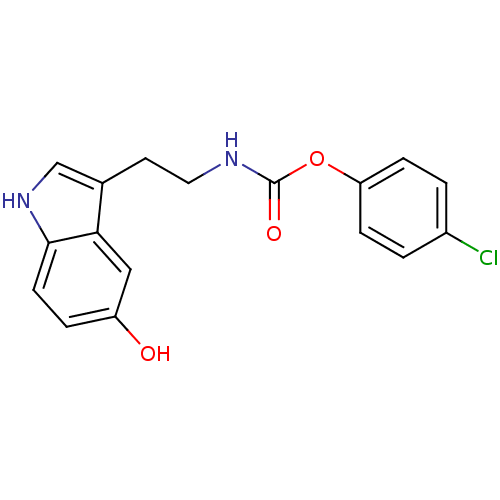 (4-chlorophenyl N-[2-(5-hydroxy-1H-indol-3-yl)ethyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rome La Sapienza | Assay Description The effect of the test compounds on the enzymatic hydrolysis of [14C]anandamide was evaluated by using membranes prepared from rat brain. [14C]Ethano... | J Med Chem 50: 6554-69 (2007) Article DOI: 10.1021/jm070678q BindingDB Entry DOI: 10.7270/Q2JH3JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM22987 ((5Z,8Z,11Z,14Z)-N-[2-(5-hydroxy-1H-indol-3-yl)ethy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | 9.0 | 37 |
University of Rome La Sapienza | Assay Description The effect of the test compounds on the enzymatic hydrolysis of [14C]anandamide was evaluated by using membranes prepared from rat brain. [14C]Ethano... | J Med Chem 50: 6554-69 (2007) Article DOI: 10.1021/jm070678q BindingDB Entry DOI: 10.7270/Q2JH3JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM22996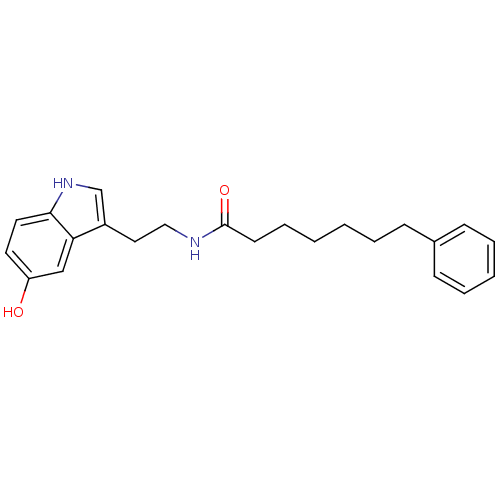 (N-[2-(5-hydroxy-1H-indol-3-yl)ethyl]-7-phenylhepta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rome La Sapienza | Assay Description The antagonistic effects of test compounds were evaluated using HEK293 cells stably overexpressing human TRPV1. Experiments were carried out by measu... | J Med Chem 50: 6554-69 (2007) Article DOI: 10.1021/jm070678q BindingDB Entry DOI: 10.7270/Q2JH3JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM22989 ((9Z,12Z,15Z)-N-[2-(5-hydroxy-1H-indol-3-yl)ethyl]o...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | 9.0 | 37 |
University of Rome La Sapienza | Assay Description The effect of the test compounds on the enzymatic hydrolysis of [14C]anandamide was evaluated by using membranes prepared from rat brain. [14C]Ethano... | J Med Chem 50: 6554-69 (2007) Article DOI: 10.1021/jm070678q BindingDB Entry DOI: 10.7270/Q2JH3JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM22990 ((6Z,9Z,12Z)-N-[2-(5-hydroxy-1H-indol-3-yl)ethyl]oc...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | 9.0 | 37 |
University of Rome La Sapienza | Assay Description The effect of the test compounds on the enzymatic hydrolysis of [14C]anandamide was evaluated by using membranes prepared from rat brain. [14C]Ethano... | J Med Chem 50: 6554-69 (2007) Article DOI: 10.1021/jm070678q BindingDB Entry DOI: 10.7270/Q2JH3JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM23026 (4-(benzyloxy)phenyl N-[2-(5-hydroxy-1H-indol-3-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
University of Rome La Sapienza | Assay Description The antagonistic effects of test compounds were evaluated using HEK293 cells stably overexpressing human TRPV1. Experiments were carried out by measu... | J Med Chem 50: 6554-69 (2007) Article DOI: 10.1021/jm070678q BindingDB Entry DOI: 10.7270/Q2JH3JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM23025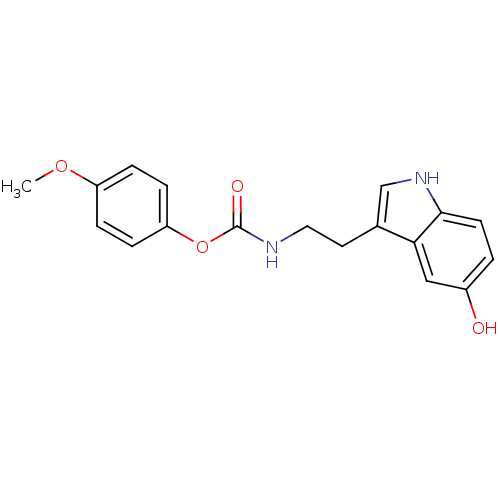 (4-methoxyphenyl N-[2-(5-hydroxy-1H-indol-3-yl)ethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
University of Rome La Sapienza | Assay Description The antagonistic effects of test compounds were evaluated using HEK293 cells stably overexpressing human TRPV1. Experiments were carried out by measu... | J Med Chem 50: 6554-69 (2007) Article DOI: 10.1021/jm070678q BindingDB Entry DOI: 10.7270/Q2JH3JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM23024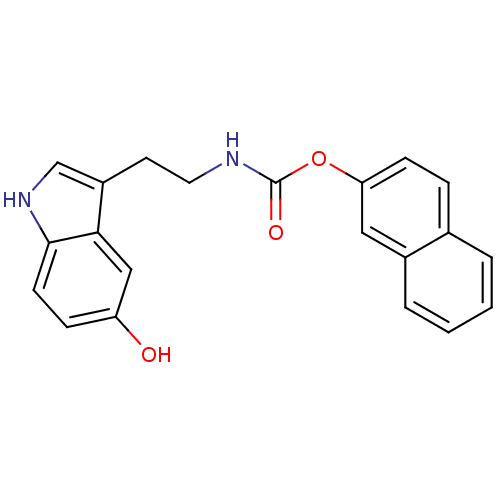 (N-arachidonoylserotonin carbamate analogue, 3o | n...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
University of Rome La Sapienza | Assay Description The antagonistic effects of test compounds were evaluated using HEK293 cells stably overexpressing human TRPV1. Experiments were carried out by measu... | J Med Chem 50: 6554-69 (2007) Article DOI: 10.1021/jm070678q BindingDB Entry DOI: 10.7270/Q2JH3JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM23023 (4-iodophenyl N-[2-(5-hydroxy-1H-indol-3-yl)ethyl]c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
University of Rome La Sapienza | Assay Description The antagonistic effects of test compounds were evaluated using HEK293 cells stably overexpressing human TRPV1. Experiments were carried out by measu... | J Med Chem 50: 6554-69 (2007) Article DOI: 10.1021/jm070678q BindingDB Entry DOI: 10.7270/Q2JH3JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM23022 (3-iodophenyl N-[2-(5-hydroxy-1H-indol-3-yl)ethyl]c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
University of Rome La Sapienza | Assay Description The antagonistic effects of test compounds were evaluated using HEK293 cells stably overexpressing human TRPV1. Experiments were carried out by measu... | J Med Chem 50: 6554-69 (2007) Article DOI: 10.1021/jm070678q BindingDB Entry DOI: 10.7270/Q2JH3JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM23021 (4-chlorophenyl N-[2-(5-hydroxy-1H-indol-3-yl)ethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
University of Rome La Sapienza | Assay Description The antagonistic effects of test compounds were evaluated using HEK293 cells stably overexpressing human TRPV1. Experiments were carried out by measu... | J Med Chem 50: 6554-69 (2007) Article DOI: 10.1021/jm070678q BindingDB Entry DOI: 10.7270/Q2JH3JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM23020 (3-chlorophenyl N-[2-(5-hydroxy-1H-indol-3-yl)ethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
University of Rome La Sapienza | Assay Description The antagonistic effects of test compounds were evaluated using HEK293 cells stably overexpressing human TRPV1. Experiments were carried out by measu... | J Med Chem 50: 6554-69 (2007) Article DOI: 10.1021/jm070678q BindingDB Entry DOI: 10.7270/Q2JH3JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 118 total ) | Next | Last >> |