 Found 497 hits with Last Name = 'wegener' and Initial = 'a'
Found 497 hits with Last Name = 'wegener' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50524862
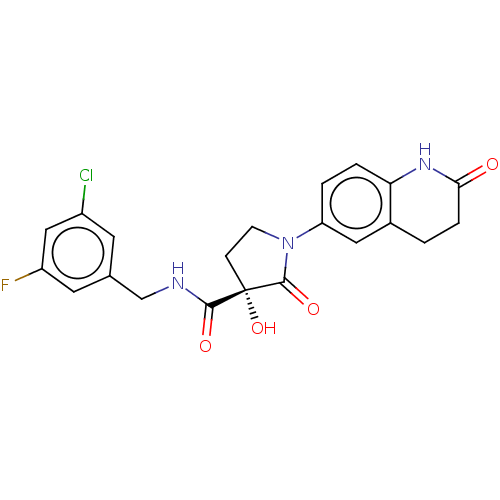
(CHEMBL4475680)Show SMILES O[C@@]1(CCN(C1=O)c1ccc2NC(=O)CCc2c1)C(=O)NCc1cc(F)cc(Cl)c1 |r| Show InChI InChI=1S/C21H19ClFN3O4/c22-14-7-12(8-15(23)10-14)11-24-19(28)21(30)5-6-26(20(21)29)16-2-3-17-13(9-16)1-4-18(27)25-17/h2-3,7-10,30H,1,4-6,11H2,(H,24,28)(H,25,27)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare
Curated by ChEMBL
| Assay Description
Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b... |
J Med Chem 62: 11119-11134 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01070
BindingDB Entry DOI: 10.7270/Q26H4MXQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50524862

(CHEMBL4475680)Show SMILES O[C@@]1(CCN(C1=O)c1ccc2NC(=O)CCc2c1)C(=O)NCc1cc(F)cc(Cl)c1 |r| Show InChI InChI=1S/C21H19ClFN3O4/c22-14-7-12(8-15(23)10-14)11-24-19(28)21(30)5-6-26(20(21)29)16-2-3-17-13(9-16)1-4-18(27)25-17/h2-3,7-10,30H,1,4-6,11H2,(H,24,28)(H,25,27)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare
Curated by ChEMBL
| Assay Description
Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b... |
J Med Chem 62: 11119-11134 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01070
BindingDB Entry DOI: 10.7270/Q26H4MXQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM401307

(US10005756, Compound A78)Show SMILES O[C@@]1(CCN(C1=O)c1cnc2[nH]ccc2c1)C(=O)NCc1cc(F)cc(F)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare
Curated by ChEMBL
| Assay Description
Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b... |
J Med Chem 62: 11119-11134 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01070
BindingDB Entry DOI: 10.7270/Q26H4MXQ |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM401307

(US10005756, Compound A78)Show SMILES O[C@@]1(CCN(C1=O)c1cnc2[nH]ccc2c1)C(=O)NCc1cc(F)cc(F)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare
Curated by ChEMBL
| Assay Description
Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b... |
J Med Chem 62: 11119-11134 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01070
BindingDB Entry DOI: 10.7270/Q26H4MXQ |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50531161

(CHEMBL4448724)Show SMILES O[C@@]1(CCN(C1=O)c1cnc2[nH]ccc2c1)C(=O)NCc1cc(F)cc(Cl)c1 |r| Show InChI InChI=1S/C19H16ClFN4O3/c20-13-5-11(6-14(21)8-13)9-24-17(26)19(28)2-4-25(18(19)27)15-7-12-1-3-22-16(12)23-10-15/h1,3,5-8,10,28H,2,4,9H2,(H,22,23)(H,24,26)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare
Curated by ChEMBL
| Assay Description
Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b... |
J Med Chem 62: 11119-11134 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01070
BindingDB Entry DOI: 10.7270/Q26H4MXQ |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50531161

(CHEMBL4448724)Show SMILES O[C@@]1(CCN(C1=O)c1cnc2[nH]ccc2c1)C(=O)NCc1cc(F)cc(Cl)c1 |r| Show InChI InChI=1S/C19H16ClFN4O3/c20-13-5-11(6-14(21)8-13)9-24-17(26)19(28)2-4-25(18(19)27)15-7-12-1-3-22-16(12)23-10-15/h1,3,5-8,10,28H,2,4,9H2,(H,22,23)(H,24,26)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare
Curated by ChEMBL
| Assay Description
Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b... |
J Med Chem 62: 11119-11134 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01070
BindingDB Entry DOI: 10.7270/Q26H4MXQ |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50531163
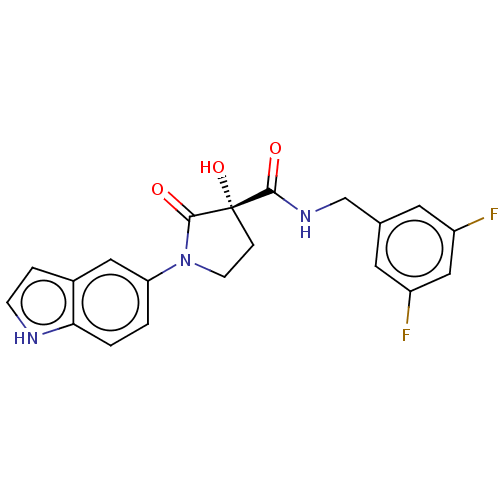
(CHEMBL4464946)Show SMILES O[C@@]1(CCN(C1=O)c1ccc2[nH]ccc2c1)C(=O)NCc1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C20H17F2N3O3/c21-14-7-12(8-15(22)10-14)11-24-18(26)20(28)4-6-25(19(20)27)16-1-2-17-13(9-16)3-5-23-17/h1-3,5,7-10,23,28H,4,6,11H2,(H,24,26)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare
Curated by ChEMBL
| Assay Description
Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b... |
J Med Chem 62: 11119-11134 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01070
BindingDB Entry DOI: 10.7270/Q26H4MXQ |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50531163

(CHEMBL4464946)Show SMILES O[C@@]1(CCN(C1=O)c1ccc2[nH]ccc2c1)C(=O)NCc1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C20H17F2N3O3/c21-14-7-12(8-15(22)10-14)11-24-18(26)20(28)4-6-25(19(20)27)16-1-2-17-13(9-16)3-5-23-17/h1-3,5,7-10,23,28H,4,6,11H2,(H,24,26)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare
Curated by ChEMBL
| Assay Description
Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b... |
J Med Chem 62: 11119-11134 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01070
BindingDB Entry DOI: 10.7270/Q26H4MXQ |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610833

(CHEMBL5276884)Show SMILES COc1ccc(c2ncccc12)S(=O)(=O)Nc1ccccc1C#Cc1cnc(C(O)=O)c(C)c1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610836

(MSC-4381)Show SMILES CCOc1ccc(c2ncccc12)S(=O)(=O)Nc1ccc(Cl)cc1C#Cc1cnc(cc1OC)C(O)=O | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610834
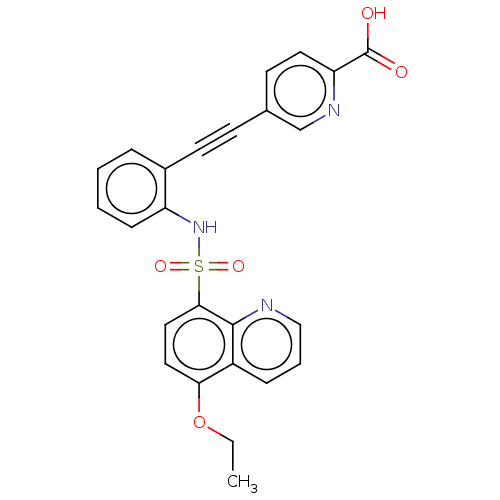
(CHEMBL5279064)Show SMILES CCOc1ccc(c2ncccc12)S(=O)(=O)Nc1ccccc1C#Cc1ccc(nc1)C(O)=O | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610835

(CHEMBL5267349)Show SMILES CCOc1ccc(c2ncccc12)S(=O)(=O)Nc1ccccc1C#Cc1cnc(cc1OC)C(O)=O | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610837
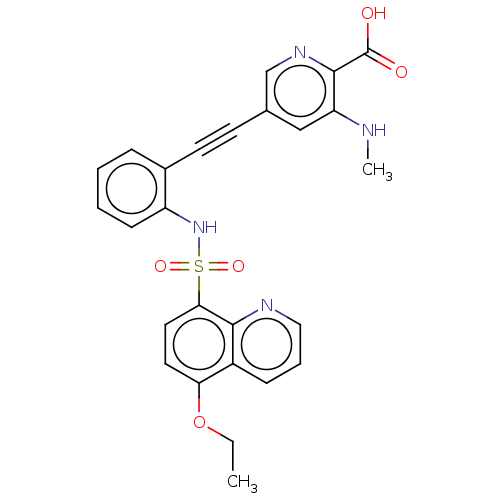
(CHEMBL5281492)Show SMILES CCOc1ccc(c2ncccc12)S(=O)(=O)Nc1ccccc1C#Cc1cnc(C(O)=O)c(NC)c1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610830
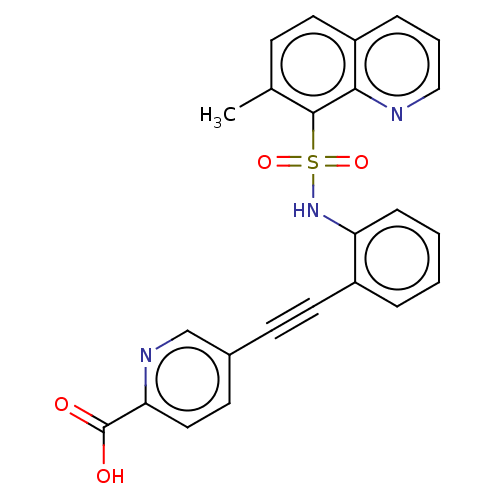
(CHEMBL5267752)Show SMILES Cc1ccc2cccnc2c1S(=O)(=O)Nc1ccccc1C#Cc1ccc(nc1)C(O)=O | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610826

(CHEMBL5287351)Show SMILES Cc1cnc2c(cccc2c1)S(=O)(=O)Nc1ccccc1C#Cc1ccc(nc1)C(O)=O | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610827
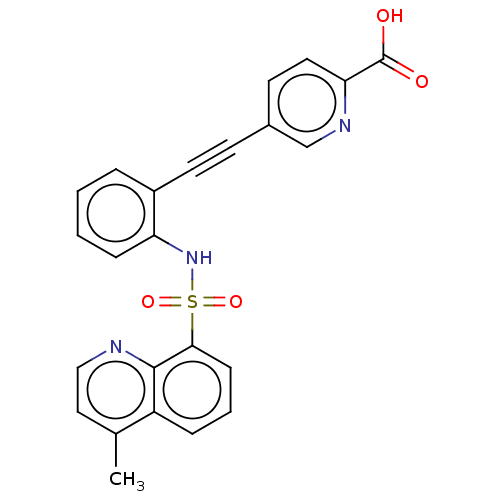
(CHEMBL5287780)Show SMILES Cc1ccnc2c(cccc12)S(=O)(=O)Nc1ccccc1C#Cc1ccc(nc1)C(O)=O | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610824

(CHEMBL5268966)Show SMILES OC(=O)c1ccc(cn1)C#Cc1ccccc1NS(=O)(=O)c1cccc2cccnc12 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610829
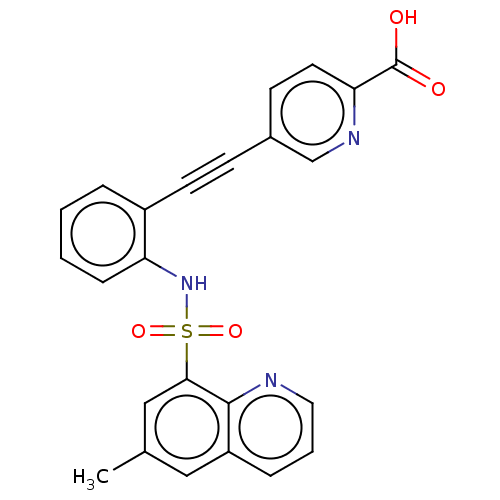
(CHEMBL5288904)Show SMILES Cc1cc(c2ncccc2c1)S(=O)(=O)Nc1ccccc1C#Cc1ccc(nc1)C(O)=O | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610831
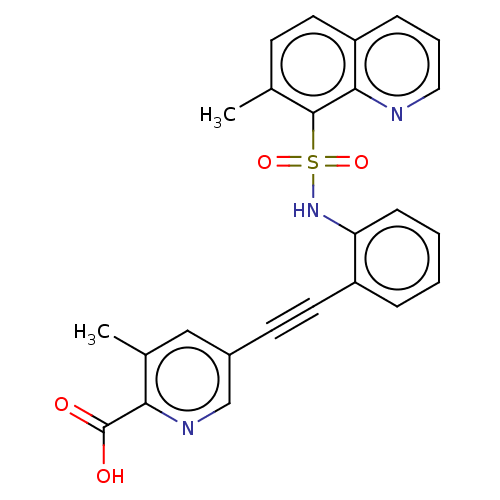
(CHEMBL5265956)Show SMILES Cc1ccc2cccnc2c1S(=O)(=O)Nc1ccccc1C#Cc1cnc(C(O)=O)c(C)c1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610825

(CHEMBL5266104)Show SMILES Cc1ccc2cccc(c2n1)S(=O)(=O)Nc1ccccc1C#Cc1ccc(nc1)C(O)=O | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610828
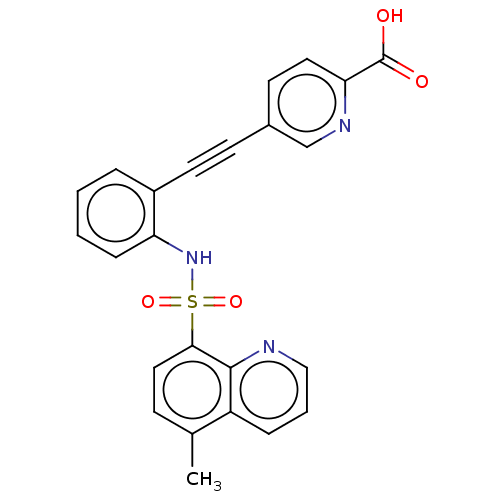
(CHEMBL5271816)Show SMILES Cc1ccc(c2ncccc12)S(=O)(=O)Nc1ccccc1C#Cc1ccc(nc1)C(O)=O | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 222 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Mus musculus) | BDBM50610868
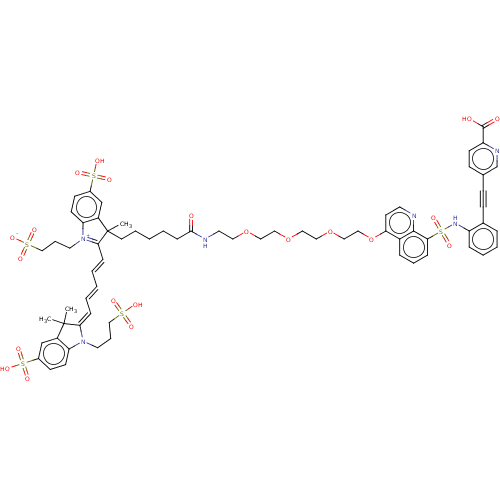
(CHEMBL5282978)Show SMILES CC1(C)\C(=C\C=C\C=C\C2=[N+](CCCS([O-])(=O)=O)c3ccc(cc3C2(C)CCCCCC(=O)NCCOCCOCCOCCOc2ccnc3c(cccc23)S(=O)(=O)Nc2ccccc2C#Cc2ccc(nc2)C(O)=O)S(O)(=O)=O)N(CCCS(O)(=O)=O)c2ccc(cc12)S(O)(=O)=O |c:9| | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 301 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM291573
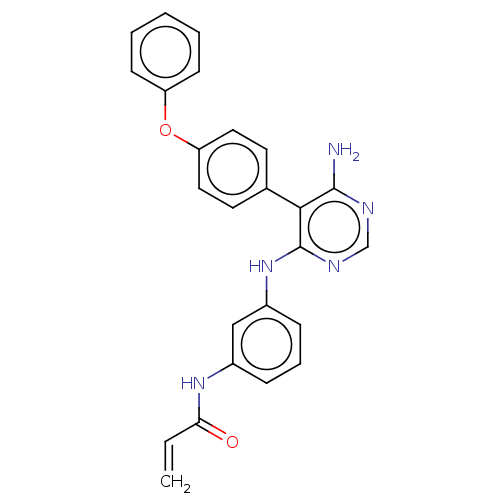
(N-(3-((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)a...)Show SMILES Nc1ncnc(Nc2cccc(NC(=O)C=C)c2)c1-c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C25H21N5O2/c1-2-22(31)29-18-7-6-8-19(15-18)30-25-23(24(26)27-16-28-25)17-11-13-21(14-12-17)32-20-9-4-3-5-10-20/h2-16H,1H2,(H,29,31)(H3,26,27,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by HPLC analysis |
J Med Chem 62: 7643-7655 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00794
BindingDB Entry DOI: 10.7270/Q21R6TXT |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM291413

(1-(6-((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)a...)Show SMILES Nc1ncnc(NC2CC3(C2)CN(C3)C(=O)C=C)c1-c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C25H25N5O2/c1-2-21(31)30-14-25(15-30)12-18(13-25)29-24-22(23(26)27-16-28-24)17-8-10-20(11-9-17)32-19-6-4-3-5-7-19/h2-11,16,18H,1,12-15H2,(H3,26,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by HPLC analysis |
J Med Chem 62: 7643-7655 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00794
BindingDB Entry DOI: 10.7270/Q21R6TXT |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM291455

(N-(4-((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)a...)Show SMILES Nc1ncnc(NC23CC(C2)(CC3)NC(=O)C=C)c1-c1ccc(Oc2ccccc2)cc1 |(-.82,-6.28,;-2.15,-5.51,;-3.48,-6.28,;-4.82,-5.51,;-4.82,-3.97,;-3.48,-3.2,;-3.48,-1.66,;-4.82,-.89,;-4.82,.65,;-6.28,1.13,;-5.88,-.36,;-7.19,-.12,;-6.28,-1.37,;-6.68,2.61,;-5.59,3.7,;-4.1,3.3,;-5.99,5.19,;-4.9,6.28,;-2.15,-3.97,;-.82,-3.2,;.52,-3.97,;1.85,-3.2,;1.85,-1.66,;3.19,-.89,;4.52,-1.66,;4.52,-3.2,;5.85,-3.97,;7.19,-3.2,;7.19,-1.66,;5.85,-.89,;.52,-.89,;-.82,-1.66,)| Show InChI InChI=1S/C25H25N5O2/c1-2-20(31)29-24-12-13-25(14-24,15-24)30-23-21(22(26)27-16-28-23)17-8-10-19(11-9-17)32-18-6-4-3-5-7-18/h2-11,16H,1,12-15H2,(H,29,31)(H3,26,27,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by HPLC analysis |
J Med Chem 62: 7643-7655 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00794
BindingDB Entry DOI: 10.7270/Q21R6TXT |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM291452
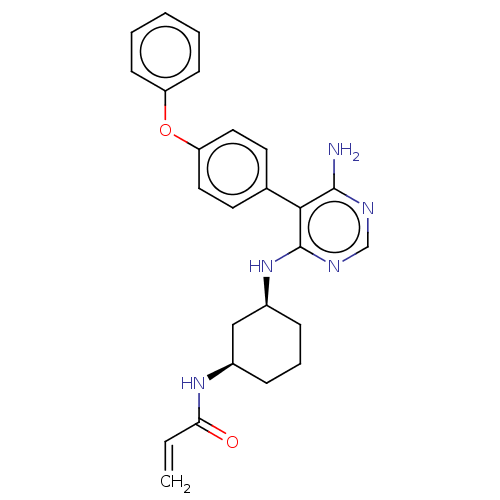
(N-((1R,3S)-3-((6-amino-5-(4-phenoxyphenyl)pyrimidi...)Show SMILES Nc1ncnc(N[C@H]2CCC[C@H](C2)NC(=O)C=C)c1-c1ccc(Oc2ccccc2)cc1 |r| Show InChI InChI=1S/C25H27N5O2/c1-2-22(31)29-18-7-6-8-19(15-18)30-25-23(24(26)27-16-28-25)17-11-13-21(14-12-17)32-20-9-4-3-5-10-20/h2-5,9-14,16,18-19H,1,6-8,15H2,(H,29,31)(H3,26,27,28,30)/t18-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by HPLC analysis |
J Med Chem 62: 7643-7655 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00794
BindingDB Entry DOI: 10.7270/Q21R6TXT |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM291522

(1-(4-(((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)...)Show SMILES Nc1ncnc(NCC2CCN(CC2)C(=O)C=C)c1-c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C25H27N5O2/c1-2-22(31)30-14-12-18(13-15-30)16-27-25-23(24(26)28-17-29-25)19-8-10-21(11-9-19)32-20-6-4-3-5-7-20/h2-11,17-18H,1,12-16H2,(H3,26,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by HPLC analysis |
J Med Chem 62: 7643-7655 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00794
BindingDB Entry DOI: 10.7270/Q21R6TXT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM291635
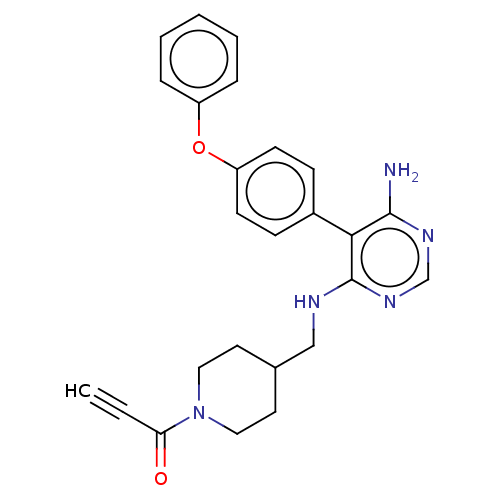
(1-(4-(((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)...)Show SMILES Nc1ncnc(NCC2CCN(CC2)C(=O)C#C)c1-c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C25H25N5O2/c1-2-22(31)30-14-12-18(13-15-30)16-27-25-23(24(26)28-17-29-25)19-8-10-21(11-9-19)32-20-6-4-3-5-7-20/h1,3-11,17-18H,12-16H2,(H3,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... |
J Med Chem 62: 7643-7655 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00794
BindingDB Entry DOI: 10.7270/Q21R6TXT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50519156
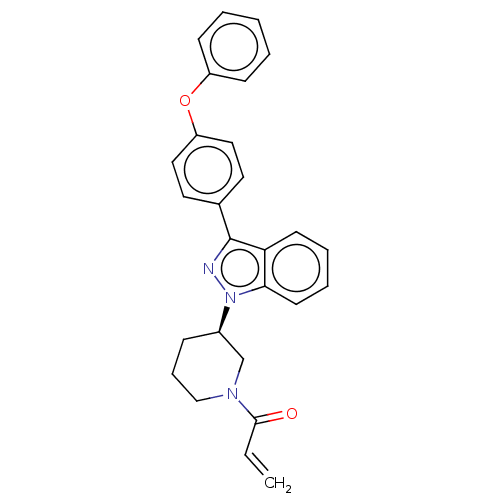
(CHEMBL4466205)Show SMILES C=CC(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2)c2ccccc12 |r| Show InChI InChI=1S/C27H25N3O2/c1-2-26(31)29-18-8-9-21(19-29)30-25-13-7-6-12-24(25)27(28-30)20-14-16-23(17-15-20)32-22-10-4-3-5-11-22/h2-7,10-17,21H,1,8-9,18-19H2/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... |
J Med Chem 62: 7643-7655 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00794
BindingDB Entry DOI: 10.7270/Q21R6TXT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM291634
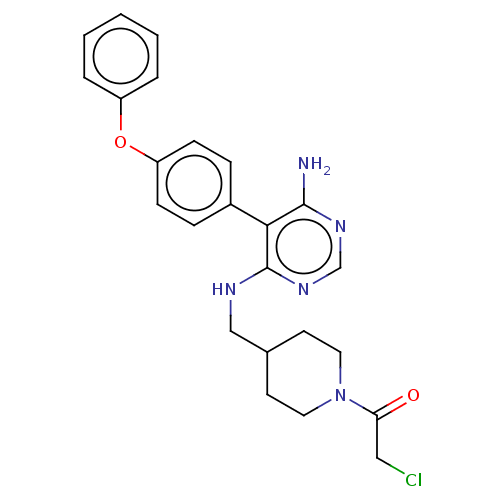
(1-(4-(((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)...)Show SMILES Nc1ncnc(NCC2CCN(CC2)C(=O)CCl)c1-c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C24H26ClN5O2/c25-14-21(31)30-12-10-17(11-13-30)15-27-24-22(23(26)28-16-29-24)18-6-8-20(9-7-18)32-19-4-2-1-3-5-19/h1-9,16-17H,10-15H2,(H3,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... |
J Med Chem 62: 7643-7655 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00794
BindingDB Entry DOI: 10.7270/Q21R6TXT |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-1
(Homo sapiens (Human)) | BDBM50593627
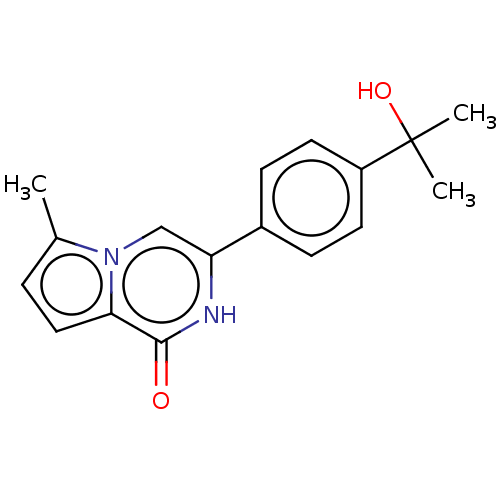
(CHEMBL5199558) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) from rat liver |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-1
(Homo sapiens (Human)) | BDBM50409622

(CHEMBL5272750)Show SMILES Cc1cccc(CN2CC(CCN3CC(C3)N3CCOCC3)(CCC2=O)c2ccc(Cl)c(Cl)c2)c1 Show InChI InChI=1S/C28H35Cl2N3O2/c1-21-3-2-4-22(15-21)17-33-20-28(8-7-27(33)34,23-5-6-25(29)26(30)16-23)9-10-31-18-24(19-31)32-11-13-35-14-12-32/h2-6,15-16,24H,7-14,17-20H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of Neisseria gonorrhoea |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-1
(Homo sapiens (Human)) | BDBM50409634

(CHEMBL5274873)Show SMILES Clc1ccc(cc1Cl)C1(CCN2CC(C2)N2CCOCC2)CCC(=O)N(CC2CC2)C1 Show InChI InChI=1S/C24H33Cl2N3O2/c25-21-4-3-19(13-22(21)26)24(6-5-23(30)29(17-24)14-18-1-2-18)7-8-27-15-20(16-27)28-9-11-31-12-10-28/h3-4,13,18,20H,1-2,5-12,14-17H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of Plasmodium berghei |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM291389
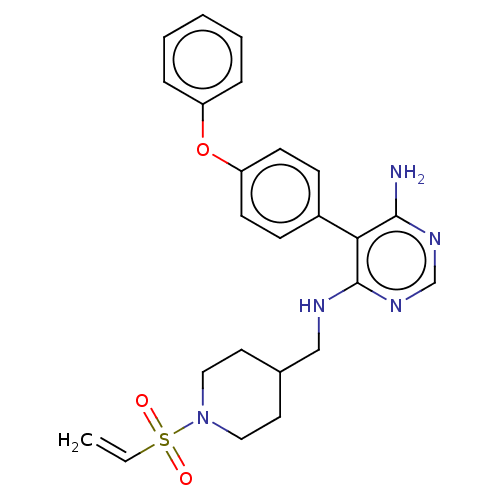
(5-(4-phenoxyphenyl)-N4-((1-(vinylsulfonyl)piperidi...)Show SMILES Nc1ncnc(NCC2CCN(CC2)S(=O)(=O)C=C)c1-c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C24H27N5O3S/c1-2-33(30,31)29-14-12-18(13-15-29)16-26-24-22(23(25)27-17-28-24)19-8-10-21(11-9-19)32-20-6-4-3-5-7-20/h2-11,17-18H,1,12-16H2,(H3,25,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... |
J Med Chem 62: 7643-7655 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00794
BindingDB Entry DOI: 10.7270/Q21R6TXT |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | BDBM50409634

(CHEMBL5274873)Show SMILES Clc1ccc(cc1Cl)C1(CCN2CC(C2)N2CCOCC2)CCC(=O)N(CC2CC2)C1 Show InChI InChI=1S/C24H33Cl2N3O2/c25-21-4-3-19(13-22(21)26)24(6-5-23(30)29(17-24)14-18-1-2-18)7-8-27-15-20(16-27)28-9-11-31-12-10-28/h3-4,13,18,20H,1-2,5-12,14-17H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of Neisseria gonorrhoea |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | BDBM50593627

(CHEMBL5199558) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) from Escherichia coli |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM291512

(N-(3-(4-amino-6-((4-phenoxyphenyl)amino)pyrimidin-...)Show SMILES Nc1ncnc(Nc2ccc(Oc3ccccc3)cc2)c1-c1cccc(NC(=O)C=C)c1 Show InChI InChI=1S/C25H21N5O2/c1-2-22(31)29-19-8-6-7-17(15-19)23-24(26)27-16-28-25(23)30-18-11-13-21(14-12-18)32-20-9-4-3-5-10-20/h2-16H,1H2,(H,29,31)(H3,26,27,28,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged human EGFR (696 to end aminoacids) expressed in baculovirus infected Sf21 cells |
J Med Chem 62: 7643-7655 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00794
BindingDB Entry DOI: 10.7270/Q21R6TXT |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | BDBM50409622

(CHEMBL5272750)Show SMILES Cc1cccc(CN2CC(CCN3CC(C3)N3CCOCC3)(CCC2=O)c2ccc(Cl)c(Cl)c2)c1 Show InChI InChI=1S/C28H35Cl2N3O2/c1-21-3-2-4-22(15-21)17-33-20-28(8-7-27(33)34,23-5-6-25(29)26(30)16-23)9-10-31-18-24(19-31)32-11-13-35-14-12-32/h2-6,15-16,24H,7-14,17-20H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) from rat liver |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-1
(Homo sapiens (Human)) | BDBM50409619

(CHEMBL5271127)Show SMILES Clc1ccc(cc1Cl)C1(CCN2CC(C2)N2CCOCC2=O)CCC(=O)N(Cc2ccccc2)C1 Show InChI InChI=1S/C27H31Cl2N3O3/c28-23-7-6-21(14-24(23)29)27(9-8-25(33)31(19-27)15-20-4-2-1-3-5-20)10-11-30-16-22(17-30)32-12-13-35-18-26(32)34/h1-7,14,22H,8-13,15-19H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) from rat liver |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | BDBM50409619

(CHEMBL5271127)Show SMILES Clc1ccc(cc1Cl)C1(CCN2CC(C2)N2CCOCC2=O)CCC(=O)N(Cc2ccccc2)C1 Show InChI InChI=1S/C27H31Cl2N3O3/c28-23-7-6-21(14-24(23)29)27(9-8-25(33)31(19-27)15-20-4-2-1-3-5-20)10-11-30-16-22(17-30)32-12-13-35-18-26(32)34/h1-7,14,22H,8-13,15-19H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of Neisseria gonorrhoea |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-1
(Homo sapiens (Human)) | BDBM50409626

(CHEMBL5284314)Show SMILES OC1CCCN(C1)C1CN(CCC2(CCC(=O)N(Cc3ccccc3)C2)c2ccc(Cl)c(Cl)c2)C1 Show InChI InChI=1S/C28H35Cl2N3O2/c29-25-9-8-22(15-26(25)30)28(11-10-27(35)33(20-28)16-21-5-2-1-3-6-21)12-14-31-17-23(18-31)32-13-4-7-24(34)19-32/h1-3,5-6,8-9,15,23-24,34H,4,7,10-14,16-20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of Plasmodium berghei |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-1
(Homo sapiens (Human)) | BDBM50593627

(CHEMBL5199558) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) from Escherichia coli |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | BDBM50593627

(CHEMBL5199558) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of Plasmodium berghei |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | BDBM50409632

(CHEMBL5279709)Show SMILES NS(=O)(=O)N1CCN(CC1)C1CN(CCC2(CCC(=O)N(CC3CC3)C2)c2ccc(Cl)c(Cl)c2)C1 Show InChI InChI=1S/C24H35Cl2N5O3S/c25-21-4-3-19(13-22(21)26)24(6-5-23(32)30(17-24)14-18-1-2-18)7-8-28-15-20(16-28)29-9-11-31(12-10-29)35(27,33)34/h3-4,13,18,20H,1-2,5-12,14-17H2,(H2,27,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) from Escherichia coli |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | BDBM50409618

(CHEMBL5281076)Show SMILES Clc1ccc(cc1Cl)C1(CCN2CC(C2)N2CCNCC2)CCC(=O)N(Cc2ccccc2)C1 Show InChI InChI=1S/C27H34Cl2N4O/c28-24-7-6-22(16-25(24)29)27(10-13-31-18-23(19-31)32-14-11-30-12-15-32)9-8-26(34)33(20-27)17-21-4-2-1-3-5-21/h1-7,16,23,30H,8-15,17-20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) from rat liver |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | BDBM50409623

(CHEMBL5280682)Show SMILES CCOC1CCN(CC1)C1CN(CCC2(CCC(=O)N(CC3CC3)C2)c2ccc(Cl)c(Cl)c2)C1 Show InChI InChI=1S/C27H39Cl2N3O2/c1-2-34-23-8-12-31(13-9-23)22-17-30(18-22)14-11-27(21-5-6-24(28)25(29)15-21)10-7-26(33)32(19-27)16-20-3-4-20/h5-6,15,20,22-23H,2-4,7-14,16-19H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of Neisseria gonorrhoea |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | BDBM50409621

(CHEMBL5269584)Show SMILES Clc1ccc(cc1Cl)C1(CCN2CC(C2)N2CCOCC2)CCC(=O)N(C1)C1CCCCC1 Show InChI InChI=1S/C26H37Cl2N3O2/c27-23-7-6-20(16-24(23)28)26(9-8-25(32)31(19-26)21-4-2-1-3-5-21)10-11-29-17-22(18-29)30-12-14-33-15-13-30/h6-7,16,21-22H,1-5,8-15,17-19H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) from rat liver |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | BDBM50409620

(CHEMBL5275576)Show SMILES Clc1ccc(cc1Cl)C1(CCN2CC(C2)N2CCS(=O)CC2)CCC(=O)N(Cc2ccccc2)C1 Show InChI InChI=1S/C27H33Cl2N3O2S/c28-24-7-6-22(16-25(24)29)27(9-8-26(33)32(20-27)17-21-4-2-1-3-5-21)10-11-30-18-23(19-30)31-12-14-35(34)15-13-31/h1-7,16,23H,8-15,17-20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of Neisseria gonorrhoea |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-1
(Homo sapiens (Human)) | BDBM50409629

(CHEMBL5269897)Show SMILES Clc1ccc(cc1Cl)C1(CCN2CC(C2)N2CCOCC2)CCC(=O)N(Cc2ccccc2)C1 Show InChI InChI=1S/C27H33Cl2N3O2/c28-24-7-6-22(16-25(24)29)27(10-11-30-18-23(19-30)31-12-14-34-15-13-31)9-8-26(33)32(20-27)17-21-4-2-1-3-5-21/h1-7,16,23H,8-15,17-20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of Neisseria gonorrhoea |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-1
(Homo sapiens (Human)) | BDBM50409621

(CHEMBL5269584)Show SMILES Clc1ccc(cc1Cl)C1(CCN2CC(C2)N2CCOCC2)CCC(=O)N(C1)C1CCCCC1 Show InChI InChI=1S/C26H37Cl2N3O2/c27-23-7-6-20(16-24(23)28)26(9-8-25(32)31(19-26)21-4-2-1-3-5-21)10-11-29-17-22(18-29)30-12-14-33-15-13-30/h6-7,16,21-22H,1-5,8-15,17-19H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of Neisseria gonorrhoea |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data