 Found 626 hits with Last Name = 'aronov' and Initial = 'am'
Found 626 hits with Last Name = 'aronov' and Initial = 'am' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093352
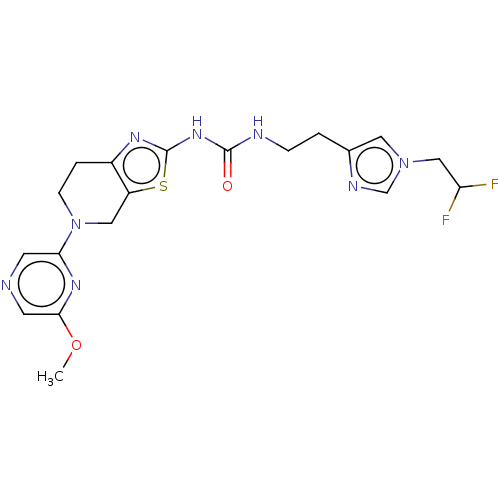
(CHEMBL3586678)Show SMILES COc1cncc(n1)N1CCc2nc(NC(=O)NCCc3cn(CC(F)F)cn3)sc2C1 Show InChI InChI=1S/C19H22F2N8O2S/c1-31-17-7-22-6-16(26-17)29-5-3-13-14(9-29)32-19(25-13)27-18(30)23-4-2-12-8-28(11-24-12)10-15(20)21/h6-8,11,15H,2-5,9-10H2,1H3,(H2,23,25,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093351
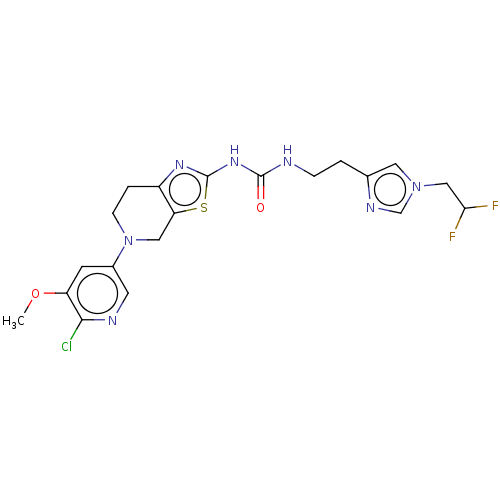
(CHEMBL3585362)Show SMILES COc1cc(cnc1Cl)N1CCc2nc(NC(=O)NCCc3cn(CC(F)F)cn3)sc2C1 Show InChI InChI=1S/C20H22ClF2N7O2S/c1-32-15-6-13(7-25-18(15)21)30-5-3-14-16(9-30)33-20(27-14)28-19(31)24-4-2-12-8-29(11-26-12)10-17(22)23/h6-8,11,17H,2-5,9-10H2,1H3,(H2,24,27,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093355
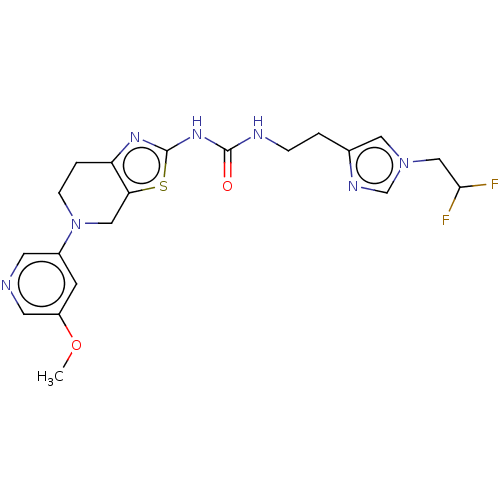
(CHEMBL3586677)Show SMILES COc1cncc(c1)N1CCc2nc(NC(=O)NCCc3cn(CC(F)F)cn3)sc2C1 Show InChI InChI=1S/C20H23F2N7O2S/c1-31-15-6-14(7-23-8-15)29-5-3-16-17(10-29)32-20(26-16)27-19(30)24-4-2-13-9-28(12-25-13)11-18(21)22/h6-9,12,18H,2-5,10-11H2,1H3,(H2,24,26,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093356
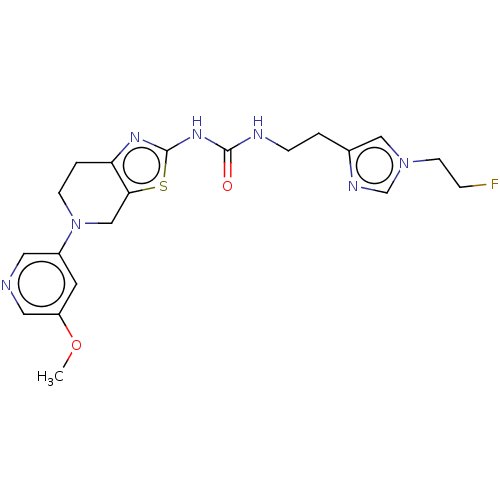
(CHEMBL3586676)Show SMILES COc1cncc(c1)N1CCc2nc(NC(=O)NCCc3cn(CCF)cn3)sc2C1 Show InChI InChI=1S/C20H24FN7O2S/c1-30-16-8-15(9-22-10-16)28-6-3-17-18(12-28)31-20(25-17)26-19(29)23-5-2-14-11-27(7-4-21)13-24-14/h8-11,13H,2-7,12H2,1H3,(H2,23,25,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093354

(CHEMBL3586679)Show SMILES COc1cncc(c1)N1CCc2nc(NC(=O)NCCc3cn(CC(F)(F)F)cn3)sc2C1 Show InChI InChI=1S/C20H22F3N7O2S/c1-32-15-6-14(7-24-8-15)30-5-3-16-17(10-30)33-19(27-16)28-18(31)25-4-2-13-9-29(12-26-13)11-20(21,22)23/h6-9,12H,2-5,10-11H2,1H3,(H2,25,27,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093395
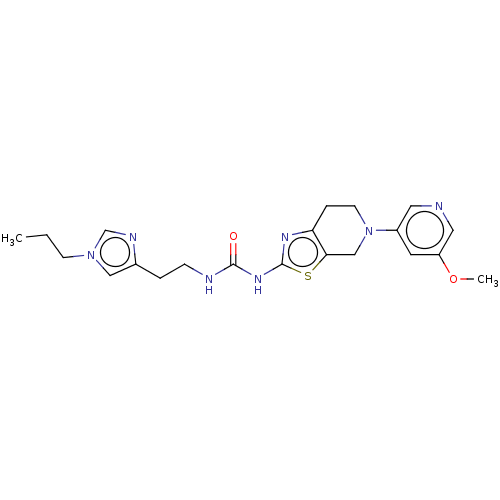
(CHEMBL3586674)Show SMILES CCCn1cnc(CCNC(=O)Nc2nc3CCN(Cc3s2)c2cncc(OC)c2)c1 Show InChI InChI=1S/C21H27N7O2S/c1-3-7-27-12-15(24-14-27)4-6-23-20(29)26-21-25-18-5-8-28(13-19(18)31-21)16-9-17(30-2)11-22-10-16/h9-12,14H,3-8,13H2,1-2H3,(H2,23,25,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093417
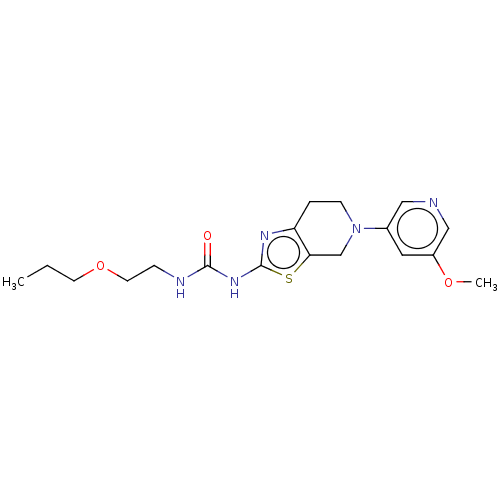
(CHEMBL3586672)Show InChI InChI=1S/C18H25N5O3S/c1-3-7-26-8-5-20-17(24)22-18-21-15-4-6-23(12-16(15)27-18)13-9-14(25-2)11-19-10-13/h9-11H,3-8,12H2,1-2H3,(H2,20,21,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093437
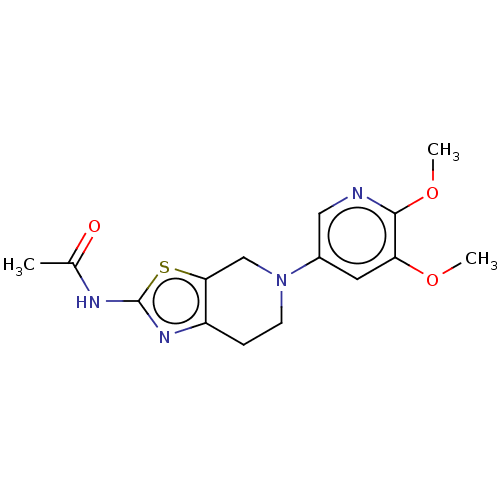
(CHEMBL3586668)Show InChI InChI=1S/C20H32O2/c1-19-7-5-14(21)10-13(19)3-4-15-16(19)6-8-20(2)17(15)9-12-11-22-18(12)20/h12-18,21H,3-11H2,1-2H3/t12-,13+,14-,15-,16+,17+,18+,19+,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093399
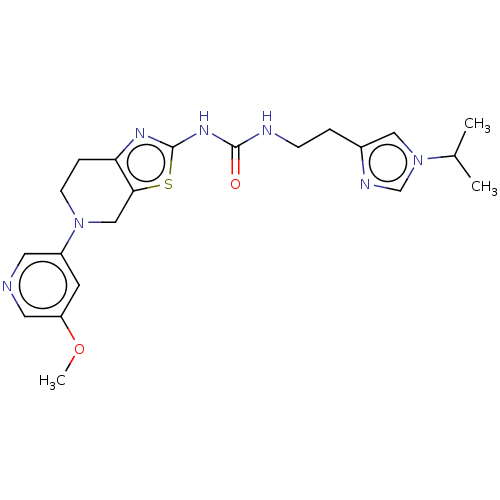
(CHEMBL3586673)Show SMILES COc1cncc(c1)N1CCc2nc(NC(=O)NCCc3cn(cn3)C(C)C)sc2C1 Show InChI InChI=1S/C21H27N7O2S/c1-14(2)28-11-15(24-13-28)4-6-23-20(29)26-21-25-18-5-7-27(12-19(18)31-21)16-8-17(30-3)10-22-9-16/h8-11,13-14H,4-7,12H2,1-3H3,(H2,23,25,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093434
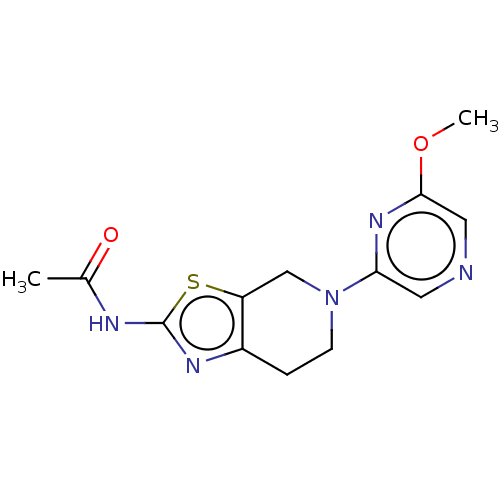
(CHEMBL3586670)Show InChI InChI=1S/C12H11NO8S2/c1-6(14)13-10-4-8(22(16,17)18)2-7-3-9(23(19,20)21)5-11(15)12(7)10/h2-5,15H,1H3,(H,13,14)(H,16,17,18)(H,19,20,21)/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093436
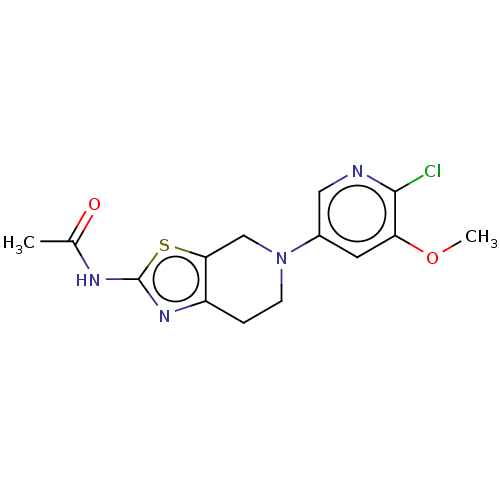
(CHEMBL3586669)Show InChI InChI=1S/C22H18O12/c23-13-5-1-11(9-15(13)25)3-7-17(27)33-19(21(29)30)20(22(31)32)34-18(28)8-4-12-2-6-14(24)16(26)10-12/h1-10,19-20,23-26H,(H,29,30)(H,31,32)/p-2/b7-3+,8-4+/t19-,20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093353
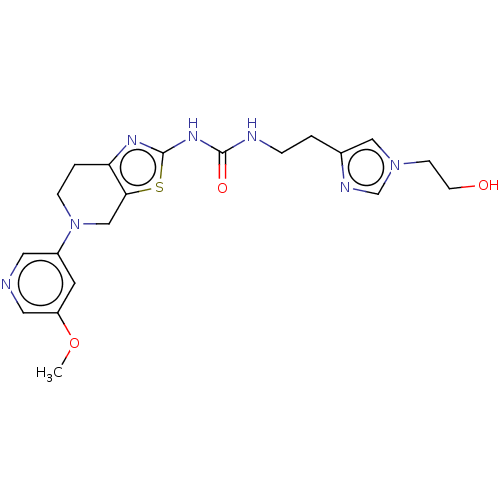
(CHEMBL3586680)Show SMILES COc1cncc(c1)N1CCc2nc(NC(=O)NCCc3cn(CCO)cn3)sc2C1 Show InChI InChI=1S/C20H25N7O3S/c1-30-16-8-15(9-21-10-16)27-5-3-17-18(12-27)31-20(24-17)25-19(29)22-4-2-14-11-26(6-7-28)13-23-14/h8-11,13,28H,2-7,12H2,1H3,(H2,22,24,25,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093391
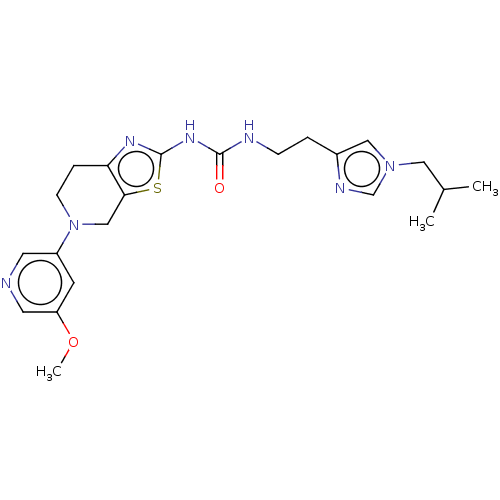
(CHEMBL3586675)Show SMILES COc1cncc(c1)N1CCc2nc(NC(=O)NCCc3cn(CC(C)C)cn3)sc2C1 Show InChI InChI=1S/C22H29N7O2S/c1-15(2)11-28-12-16(25-14-28)4-6-24-21(30)27-22-26-19-5-7-29(13-20(19)32-22)17-8-18(31-3)10-23-9-17/h8-10,12,14-15H,4-7,11,13H2,1-3H3,(H2,24,26,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093439
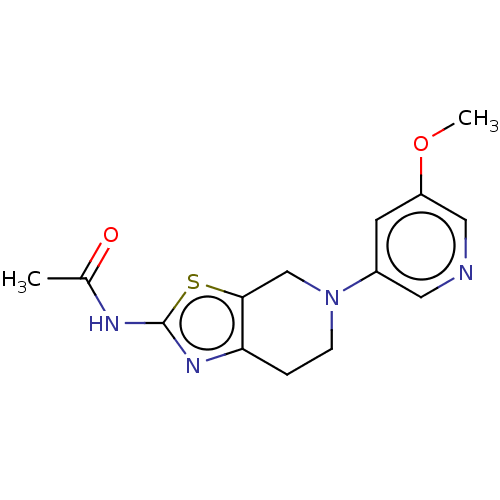
(CHEMBL3586666)Show InChI InChI=1S/C19H30O2/c1-18-7-5-12(20)9-11(18)3-4-13-14(18)6-8-19(2)15(13)10-16-17(19)21-16/h11-17,20H,3-10H2,1-2H3/t11-,12+,13+,14-,15-,16+,17+,18-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093419
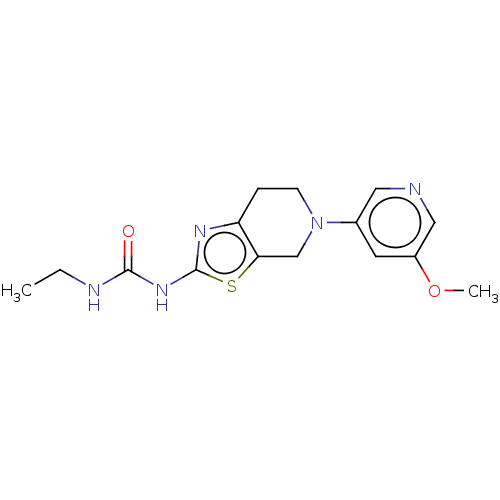
(CHEMBL3586671)Show InChI InChI=1S/C15H19N5O2S/c1-3-17-14(21)19-15-18-12-4-5-20(9-13(12)23-15)10-6-11(22-2)8-16-7-10/h6-8H,3-5,9H2,1-2H3,(H2,17,18,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093440
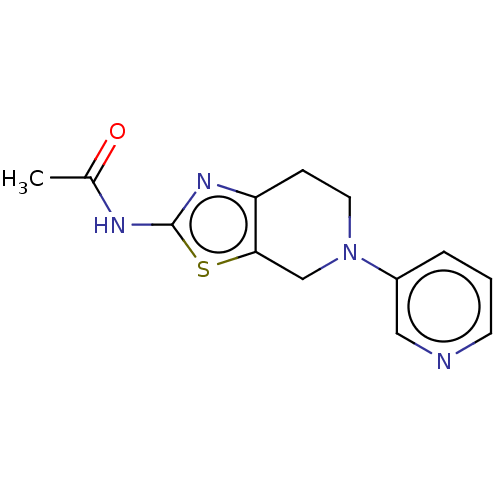
(CHEMBL3586665)Show InChI InChI=1S/C21H32O3/c1-12(22)16-6-7-17-15-5-4-13-10-14(23)8-9-20(13,2)19(15)18(24)11-21(16,17)3/h13-17,19,23H,4-11H2,1-3H3/t13?,14-,15?,16-,17?,19?,20+,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.157 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093438
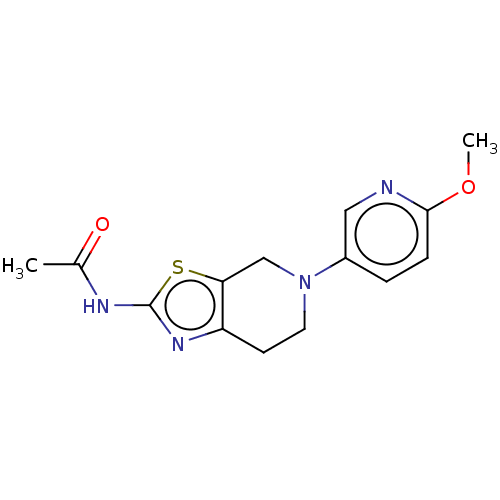
(CHEMBL3586667)Show InChI InChI=1S/C20H31NO/c1-19-9-7-15(22)11-13(19)3-5-16-17-6-4-14(12-21)20(17,2)10-8-18(16)19/h13-18,22H,3-11H2,1-2H3/t13-,14-,15+,16-,17-,18-,19-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50044290
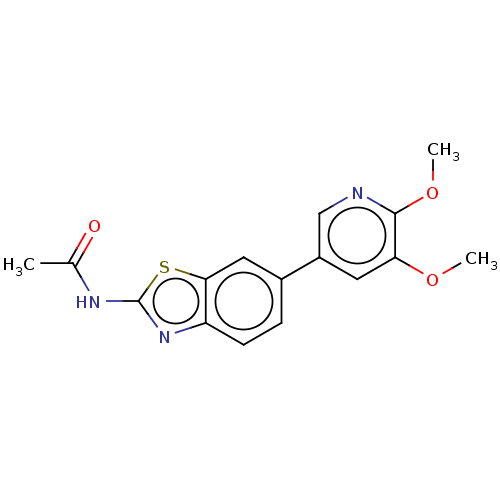
(CHEMBL3356889)Show InChI InChI=1S/C16H15N3O3S/c1-9(20)18-16-19-12-5-4-10(7-14(12)23-16)11-6-13(21-2)15(22-3)17-8-11/h4-8H,1-3H3,(H,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3K-gamma (unknown origin) using [33P]ATP as substrate after 15 mins by liquid scintillation counting analysis |
J Med Chem 58: 517-21 (2015)
Article DOI: 10.1021/jm500362j
BindingDB Entry DOI: 10.7270/Q2K35W9M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50044280
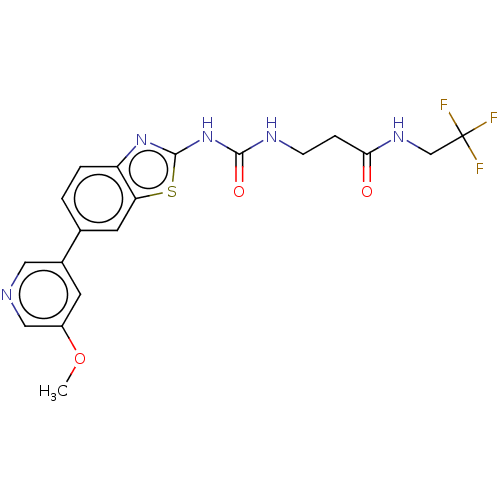
(CHEMBL3356896)Show SMILES COc1cncc(c1)-c1ccc2nc(NC(=O)NCCC(=O)NCC(F)(F)F)sc2c1 Show InChI InChI=1S/C19H18F3N5O3S/c1-30-13-6-12(8-23-9-13)11-2-3-14-15(7-11)31-18(26-14)27-17(29)24-5-4-16(28)25-10-19(20,21)22/h2-3,6-9H,4-5,10H2,1H3,(H,25,28)(H2,24,26,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3K-gamma (unknown origin) using [33P]ATP as substrate after 15 mins by liquid scintillation counting analysis |
J Med Chem 58: 517-21 (2015)
Article DOI: 10.1021/jm500362j
BindingDB Entry DOI: 10.7270/Q2K35W9M |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM35641
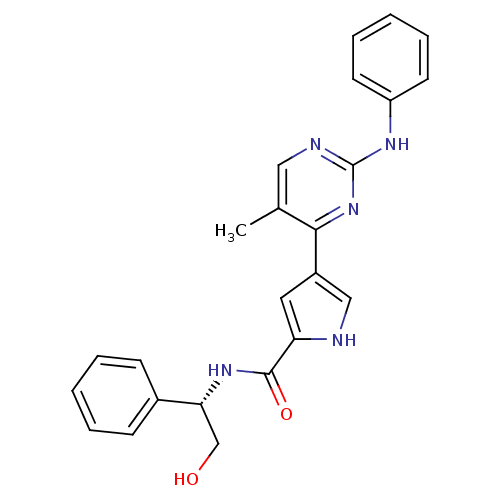
(erk000040 | pyrimidylpyrrole, 2)Show SMILES Cc1cnc(Nc2ccccc2)nc1-c1c[nH]c(c1)C(=O)N[C@H](CO)c1ccccc1 |r| Show InChI InChI=1S/C24H23N5O2/c1-16-13-26-24(27-19-10-6-3-7-11-19)29-22(16)18-12-20(25-14-18)23(31)28-21(15-30)17-8-4-2-5-9-17/h2-14,21,25,30H,15H2,1H3,(H,28,31)(H,26,27,29)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50044287
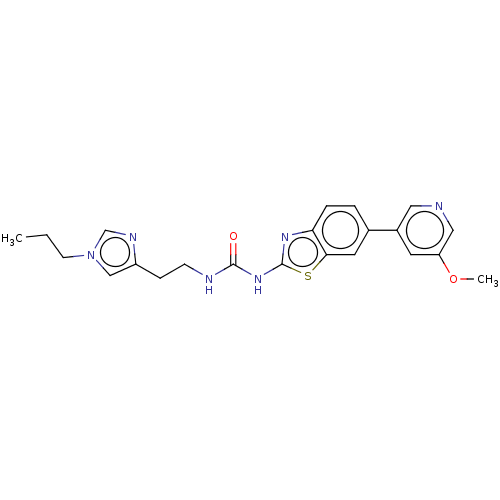
(CHEMBL3356900)Show SMILES CCCn1cnc(CCNC(=O)Nc2nc3ccc(cc3s2)-c2cncc(OC)c2)c1 Show InChI InChI=1S/C22H24N6O2S/c1-3-8-28-13-17(25-14-28)6-7-24-21(29)27-22-26-19-5-4-15(10-20(19)31-22)16-9-18(30-2)12-23-11-16/h4-5,9-14H,3,6-8H2,1-2H3,(H2,24,26,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3K-gamma (unknown origin) using [33P]ATP as substrate after 15 mins by liquid scintillation counting analysis |
J Med Chem 58: 517-21 (2015)
Article DOI: 10.1021/jm500362j
BindingDB Entry DOI: 10.7270/Q2K35W9M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM35653

(pyrimidylpyrrole, 11a)Show SMILES Cc1ccccc1Nc1ncc(C)c(n1)-c1c[nH]c(c1)C(=O)N[C@H](CO)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C25H24ClN5O2/c1-15-6-3-4-9-20(15)30-25-28-12-16(2)23(31-25)18-11-21(27-13-18)24(33)29-22(14-32)17-7-5-8-19(26)10-17/h3-13,22,27,32H,14H2,1-2H3,(H,29,33)(H,28,30,31)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM35647
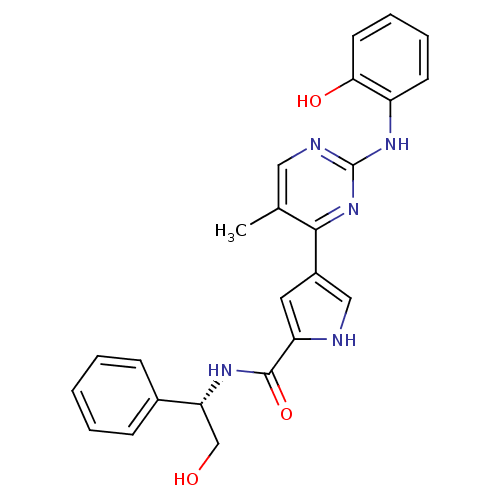
(erk000636 | pyrimidylpyrrole, 9f)Show SMILES Cc1cnc(Nc2ccccc2O)nc1-c1c[nH]c(c1)C(=O)N[C@H](CO)c1ccccc1 |r| Show InChI InChI=1S/C24H23N5O3/c1-15-12-26-24(28-18-9-5-6-10-21(18)31)29-22(15)17-11-19(25-13-17)23(32)27-20(14-30)16-7-3-2-4-8-16/h2-13,20,25,30-31H,14H2,1H3,(H,27,32)(H,26,28,29)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM35663
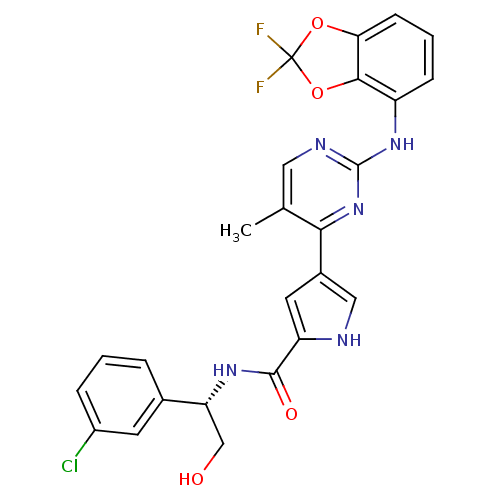
(pyrimidylpyrrole, 11k)Show SMILES Cc1cnc(Nc2cccc3OC(F)(F)Oc23)nc1-c1c[nH]c(c1)C(=O)N[C@H](CO)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C25H20ClF2N5O4/c1-13-10-30-24(32-17-6-3-7-20-22(17)37-25(27,28)36-20)33-21(13)15-9-18(29-11-15)23(35)31-19(12-34)14-4-2-5-16(26)8-14/h2-11,19,29,34H,12H2,1H3,(H,31,35)(H,30,32,33)/t19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM35662
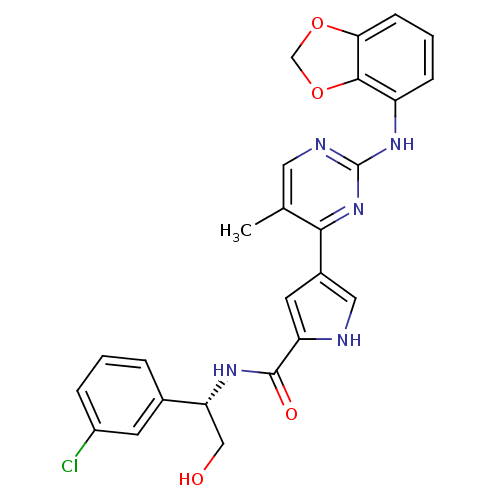
(pyrimidylpyrrole, 11j)Show SMILES Cc1cnc(Nc2cccc3OCOc23)nc1-c1c[nH]c(c1)C(=O)N[C@H](CO)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C25H22ClN5O4/c1-14-10-28-25(30-18-6-3-7-21-23(18)35-13-34-21)31-22(14)16-9-19(27-11-16)24(33)29-20(12-32)15-4-2-5-17(26)8-15/h2-11,20,27,32H,12-13H2,1H3,(H,29,33)(H,28,30,31)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM35660
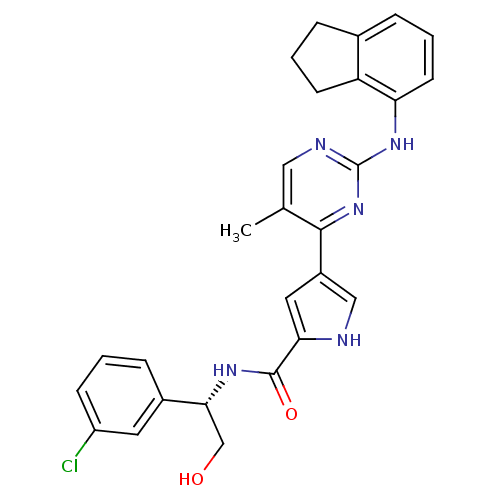
(pyrimidylpyrrole, 11h)Show SMILES Cc1cnc(Nc2cccc3CCCc23)nc1-c1c[nH]c(c1)C(=O)N[C@H](CO)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C27H26ClN5O2/c1-16-13-30-27(32-22-10-4-6-17-5-3-9-21(17)22)33-25(16)19-12-23(29-14-19)26(35)31-24(15-34)18-7-2-8-20(28)11-18/h2,4,6-8,10-14,24,29,34H,3,5,9,15H2,1H3,(H,31,35)(H,30,32,33)/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM35659
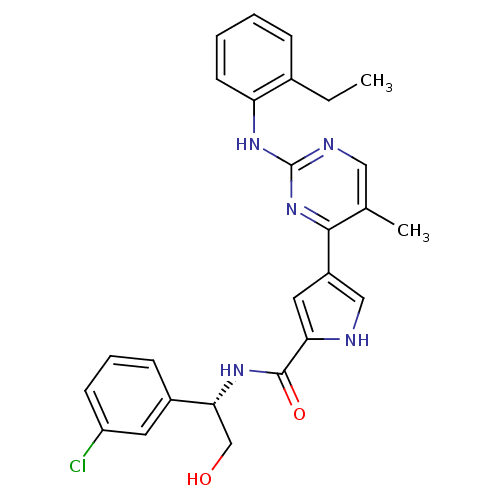
(pyrimidylpyrrole, 11g)Show SMILES CCc1ccccc1Nc1ncc(C)c(n1)-c1c[nH]c(c1)C(=O)N[C@H](CO)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C26H26ClN5O2/c1-3-17-7-4-5-10-21(17)31-26-29-13-16(2)24(32-26)19-12-22(28-14-19)25(34)30-23(15-33)18-8-6-9-20(27)11-18/h4-14,23,28,33H,3,15H2,1-2H3,(H,30,34)(H,29,31,32)/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM35657
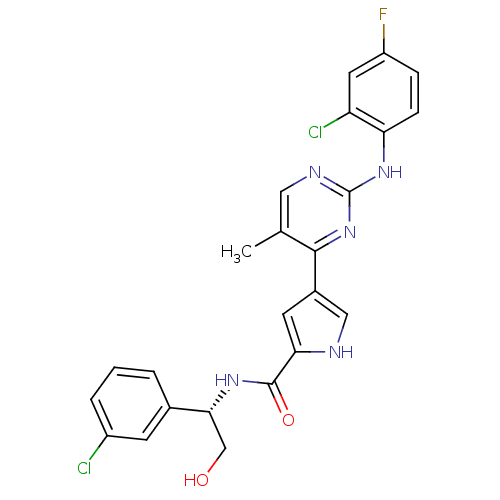
(pyrimidylpyrrole, 11e)Show SMILES Cc1cnc(Nc2ccc(F)cc2Cl)nc1-c1c[nH]c(c1)C(=O)N[C@H](CO)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C24H20Cl2FN5O2/c1-13-10-29-24(31-19-6-5-17(27)9-18(19)26)32-22(13)15-8-20(28-11-15)23(34)30-21(12-33)14-3-2-4-16(25)7-14/h2-11,21,28,33H,12H2,1H3,(H,30,34)(H,29,31,32)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM35654

(erk000526 | pyrimidylpyrrole, 11b)Show SMILES Cc1cccc(Nc2ncc(C)c(n2)-c2c[nH]c(c2)C(=O)N[C@H](CO)c2cccc(Cl)c2)c1C |r| Show InChI InChI=1S/C26H26ClN5O2/c1-15-6-4-9-21(17(15)3)31-26-29-12-16(2)24(32-26)19-11-22(28-13-19)25(34)30-23(14-33)18-7-5-8-20(27)10-18/h4-13,23,28,33H,14H2,1-3H3,(H,30,34)(H,29,31,32)/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM35653

(pyrimidylpyrrole, 11a)Show SMILES Cc1ccccc1Nc1ncc(C)c(n1)-c1c[nH]c(c1)C(=O)N[C@H](CO)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C25H24ClN5O2/c1-15-6-3-4-9-20(15)30-25-28-12-16(2)23(31-25)18-11-21(27-13-18)24(33)29-22(14-32)17-7-5-8-19(26)10-17/h3-13,22,27,32H,14H2,1-2H3,(H,29,33)(H,28,30,31)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM35651
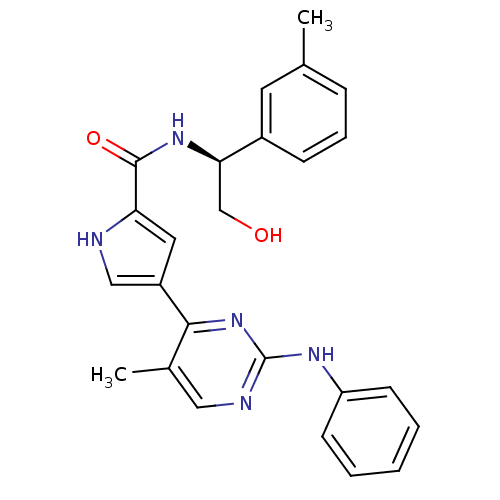
(pyrimidylpyrrole, 10d)Show SMILES Cc1cccc(c1)[C@@H](CO)NC(=O)c1cc(c[nH]1)-c1nc(Nc2ccccc2)ncc1C |r| Show InChI InChI=1S/C25H25N5O2/c1-16-7-6-8-18(11-16)22(15-31)29-24(32)21-12-19(14-26-21)23-17(2)13-27-25(30-23)28-20-9-4-3-5-10-20/h3-14,22,26,31H,15H2,1-2H3,(H,29,32)(H,27,28,30)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM35650

(pyrimidylpyrrole, 10c)Show SMILES Cc1cnc(Nc2ccccc2)nc1-c1c[nH]c(c1)C(=O)N[C@H](CO)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C24H22ClN5O2/c1-15-12-27-24(28-19-8-3-2-4-9-19)30-22(15)17-11-20(26-13-17)23(32)29-21(14-31)16-6-5-7-18(25)10-16/h2-13,21,26,31H,14H2,1H3,(H,29,32)(H,27,28,30)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM35649
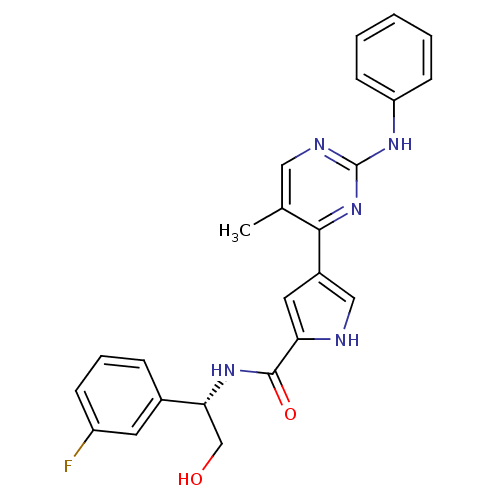
(erk000537 | pyrimidylpyrrole, 10b)Show SMILES Cc1cnc(Nc2ccccc2)nc1-c1c[nH]c(c1)C(=O)N[C@H](CO)c1cccc(F)c1 |r| Show InChI InChI=1S/C24H22FN5O2/c1-15-12-27-24(28-19-8-3-2-4-9-19)30-22(15)17-11-20(26-13-17)23(32)29-21(14-31)16-6-5-7-18(25)10-16/h2-13,21,26,31H,14H2,1H3,(H,29,32)(H,27,28,30)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM35645
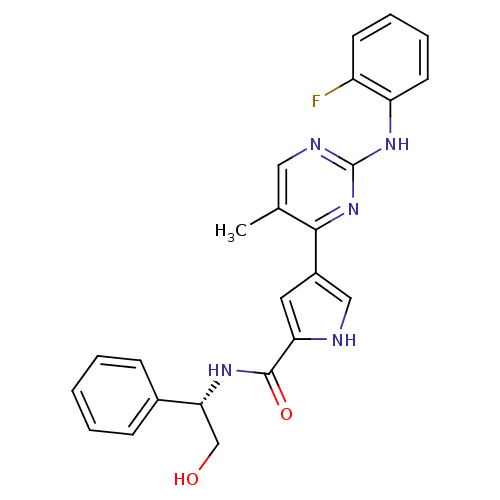
(pyrimidylpyrrole, 9d)Show SMILES Cc1cnc(Nc2ccccc2F)nc1-c1c[nH]c(c1)C(=O)N[C@H](CO)c1ccccc1 |r| Show InChI InChI=1S/C24H22FN5O2/c1-15-12-27-24(29-19-10-6-5-9-18(19)25)30-22(15)17-11-20(26-13-17)23(32)28-21(14-31)16-7-3-2-4-8-16/h2-13,21,26,31H,14H2,1H3,(H,28,32)(H,27,29,30)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM35643

(pyrimidylpyrrole, 9b)Show SMILES Cc1ccccc1Nc1ncc(C)c(n1)-c1c[nH]c(c1)C(=O)N[C@H](CO)c1ccccc1 |r| Show InChI InChI=1S/C25H25N5O2/c1-16-8-6-7-11-20(16)29-25-27-13-17(2)23(30-25)19-12-21(26-14-19)24(32)28-22(15-31)18-9-4-3-5-10-18/h3-14,22,26,31H,15H2,1-2H3,(H,28,32)(H,27,29,30)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM35642
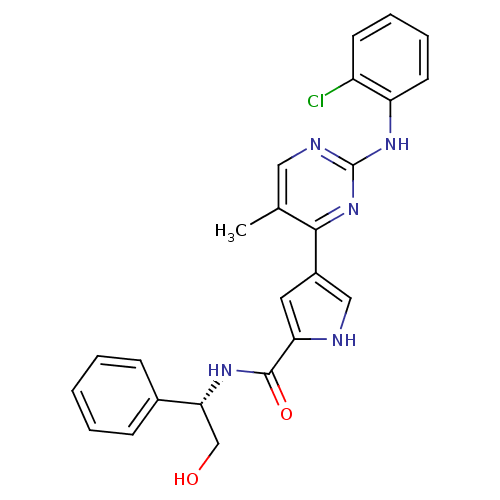
(pyrimidylpyrrole, 9a)Show SMILES Cc1cnc(Nc2ccccc2Cl)nc1-c1c[nH]c(c1)C(=O)N[C@H](CO)c1ccccc1 |r| Show InChI InChI=1S/C24H22ClN5O2/c1-15-12-27-24(29-19-10-6-5-9-18(19)25)30-22(15)17-11-20(26-13-17)23(32)28-21(14-31)16-7-3-2-4-8-16/h2-13,21,26,31H,14H2,1H3,(H,28,32)(H,27,29,30)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50044277
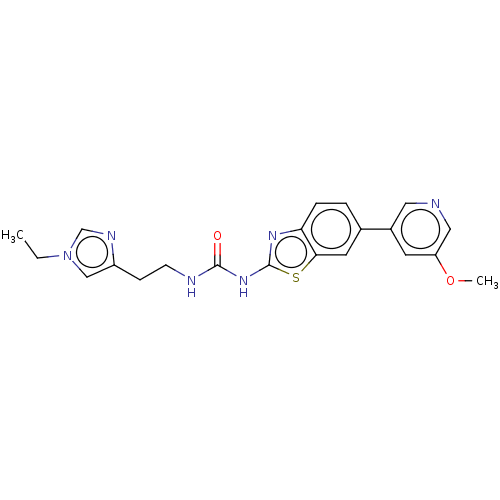
(CHEMBL3356899)Show SMILES CCn1cnc(CCNC(=O)Nc2nc3ccc(cc3s2)-c2cncc(OC)c2)c1 Show InChI InChI=1S/C21H22N6O2S/c1-3-27-12-16(24-13-27)6-7-23-20(28)26-21-25-18-5-4-14(9-19(18)30-21)15-8-17(29-2)11-22-10-15/h4-5,8-13H,3,6-7H2,1-2H3,(H2,23,25,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3K-gamma (unknown origin) using [33P]ATP as substrate after 15 mins by liquid scintillation counting analysis |
J Med Chem 58: 517-21 (2015)
Article DOI: 10.1021/jm500362j
BindingDB Entry DOI: 10.7270/Q2K35W9M |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM15645

(N-((S)-1-(3-Chloro-4-fluorophenyl)-2-hydroxyethyl)...)Show SMILES OC[C@@H](NC(=O)c1cc(c[nH]1)-c1n[nH]cc1-c1cccc(Cl)c1)c1ccc(F)c(Cl)c1 |r| Show InChI InChI=1S/C22H17Cl2FN4O2/c23-15-3-1-2-12(6-15)16-10-27-29-21(16)14-8-19(26-9-14)22(31)28-20(11-30)13-4-5-18(25)17(24)7-13/h1-10,20,26,30H,11H2,(H,27,29)(H,28,31)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by ERK2 was converted to ATP by pyruvate kinase with the production of pyruvate fr... |
J Med Chem 50: 1280-7 (2007)
Article DOI: 10.1021/jm061381f
BindingDB Entry DOI: 10.7270/Q2BR8QFV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50044287

(CHEMBL3356900)Show SMILES CCCn1cnc(CCNC(=O)Nc2nc3ccc(cc3s2)-c2cncc(OC)c2)c1 Show InChI InChI=1S/C22H24N6O2S/c1-3-8-28-13-17(25-14-28)6-7-24-21(29)27-22-26-19-5-4-15(10-20(19)31-22)16-9-18(30-2)12-23-11-16/h4-5,9-14H,3,6-8H2,1-2H3,(H2,24,26,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method |
J Med Chem 61: 5245-5256 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00085
BindingDB Entry DOI: 10.7270/Q2V98BK8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274571
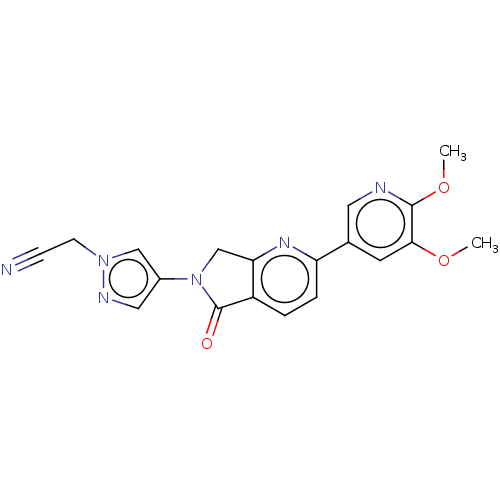
(CHEMBL4127784)Show SMILES COc1cc(cnc1OC)-c1ccc2C(=O)N(Cc2n1)c1cnn(CC#N)c1 Show InChI InChI=1S/C19H16N6O3/c1-27-17-7-12(8-21-18(17)28-2)15-4-3-14-16(23-15)11-25(19(14)26)13-9-22-24(10-13)6-5-20/h3-4,7-10H,6,11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method |
J Med Chem 61: 5245-5256 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00085
BindingDB Entry DOI: 10.7270/Q2V98BK8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM35665
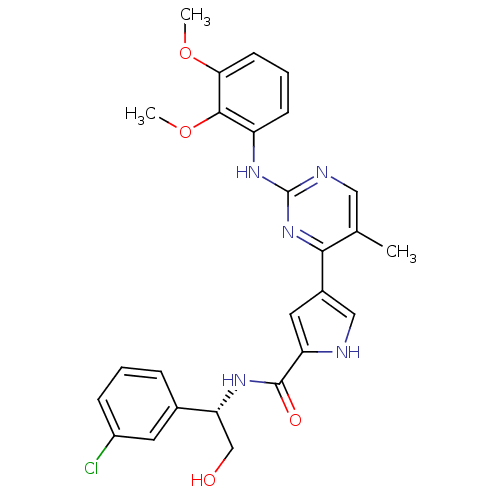
(erk000617 | pyrimidylpyrrole, 11m)Show SMILES COc1cccc(Nc2ncc(C)c(n2)-c2c[nH]c(c2)C(=O)N[C@H](CO)c2cccc(Cl)c2)c1OC |r| Show InChI InChI=1S/C26H26ClN5O4/c1-15-12-29-26(31-19-8-5-9-22(35-2)24(19)36-3)32-23(15)17-11-20(28-13-17)25(34)30-21(14-33)16-6-4-7-18(27)10-16/h4-13,21,28,33H,14H2,1-3H3,(H,30,34)(H,29,31,32)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | -49.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50549855
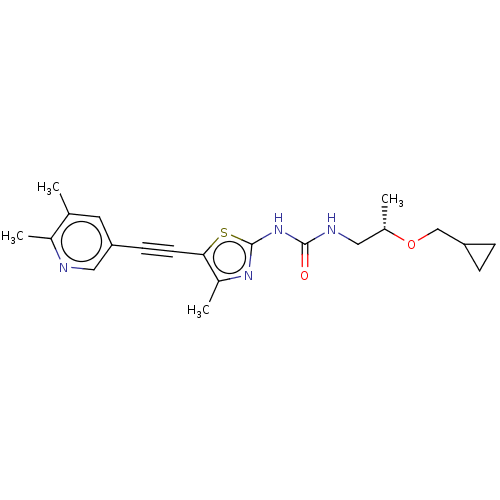
(CHEMBL4756664)Show SMILES C[C@@H](CNC(=O)Nc1nc(C)c(s1)C#Cc1cnc(C)c(C)c1)OCC1CC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate measured after 15 mins in presence of [33P]-ATP by liquid scintillation counting met... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00573
BindingDB Entry DOI: 10.7270/Q2HD8081 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50549829
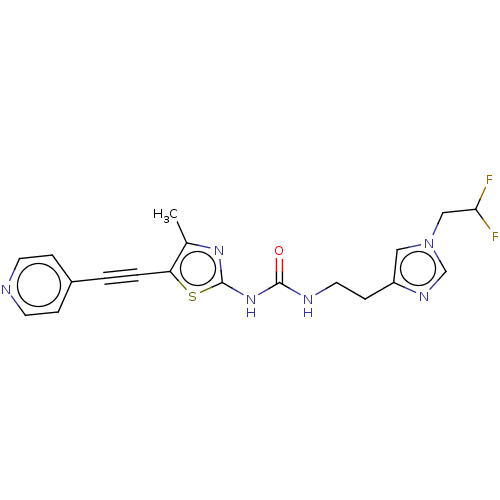
(CHEMBL4760520)Show SMILES Cc1nc(NC(=O)NCCc2cn(CC(F)F)cn2)sc1C#Cc1ccncc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate measured after 15 mins in presence of [33P]-ATP by liquid scintillation counting met... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00573
BindingDB Entry DOI: 10.7270/Q2HD8081 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50549837
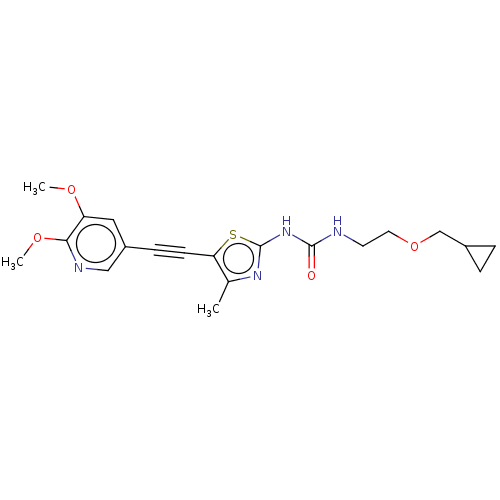
(CHEMBL4759283)Show SMILES COc1cc(cnc1OC)C#Cc1sc(NC(=O)NCCOCC2CC2)nc1C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate measured after 15 mins in presence of [33P]-ATP by liquid scintillation counting met... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00573
BindingDB Entry DOI: 10.7270/Q2HD8081 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM35644
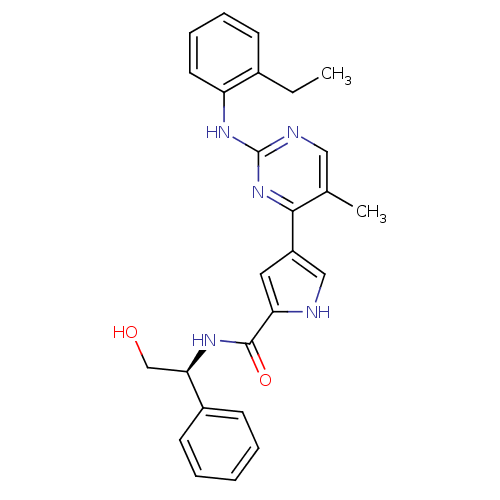
(erk000524 | pyrimidylpyrrole, 9c)Show SMILES CCc1ccccc1Nc1ncc(C)c(n1)-c1c[nH]c(c1)C(=O)N[C@H](CO)c1ccccc1 |r| Show InChI InChI=1S/C26H27N5O2/c1-3-18-9-7-8-12-21(18)30-26-28-14-17(2)24(31-26)20-13-22(27-15-20)25(33)29-23(16-32)19-10-5-4-6-11-19/h4-15,23,27,32H,3,16H2,1-2H3,(H,29,33)(H,28,30,31)/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | -49.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM35646

(erk000506 | pyrimidylpyrrole, 9e)Show SMILES Cc1cnc(Nc2ccccc2C(F)(F)F)nc1-c1c[nH]c(c1)C(=O)N[C@H](CO)c1ccccc1 |r| Show InChI InChI=1S/C25H22F3N5O2/c1-15-12-30-24(32-19-10-6-5-9-18(19)25(26,27)28)33-22(15)17-11-20(29-13-17)23(35)31-21(14-34)16-7-3-2-4-8-16/h2-13,21,29,34H,14H2,1H3,(H,31,35)(H,30,32,33)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | -48.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50044295
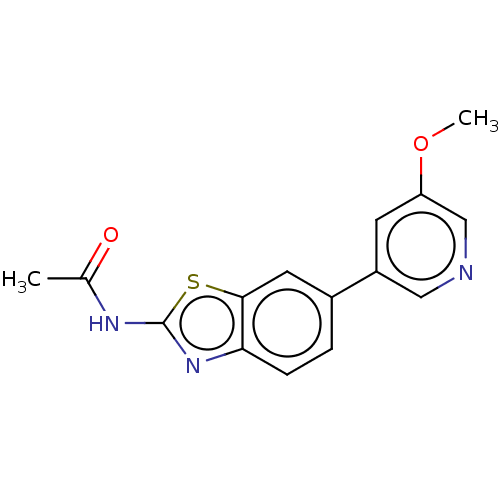
(CHEMBL3356884)Show InChI InChI=1S/C15H13N3O2S/c1-9(19)17-15-18-13-4-3-10(6-14(13)21-15)11-5-12(20-2)8-16-7-11/h3-8H,1-2H3,(H,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3K-gamma (unknown origin) using [33P]ATP as substrate after 15 mins by liquid scintillation counting analysis |
J Med Chem 58: 517-21 (2015)
Article DOI: 10.1021/jm500362j
BindingDB Entry DOI: 10.7270/Q2K35W9M |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50549828
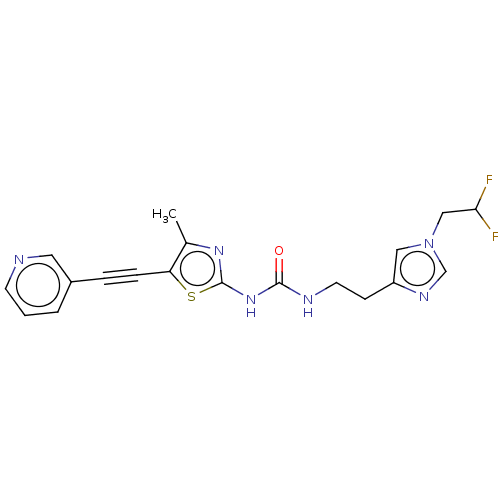
(CHEMBL4795990)Show SMILES Cc1nc(NC(=O)NCCc2cn(CC(F)F)cn2)sc1C#Cc1cccnc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate measured after 15 mins in presence of [33P]-ATP by liquid scintillation counting met... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00573
BindingDB Entry DOI: 10.7270/Q2HD8081 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50549856
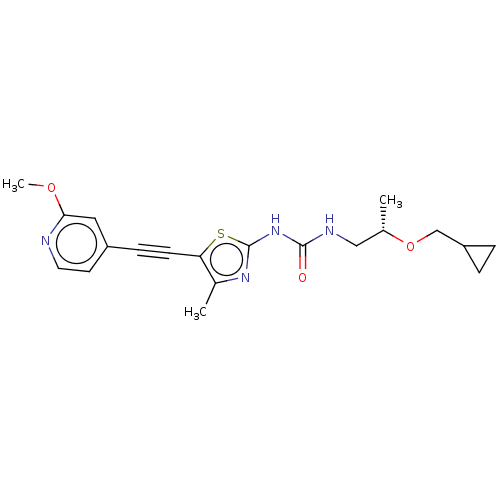
(CHEMBL4761824)Show SMILES COc1cc(ccn1)C#Cc1sc(NC(=O)NC[C@H](C)OCC2CC2)nc1C |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate measured after 15 mins in presence of [33P]-ATP by liquid scintillation counting met... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00573
BindingDB Entry DOI: 10.7270/Q2HD8081 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
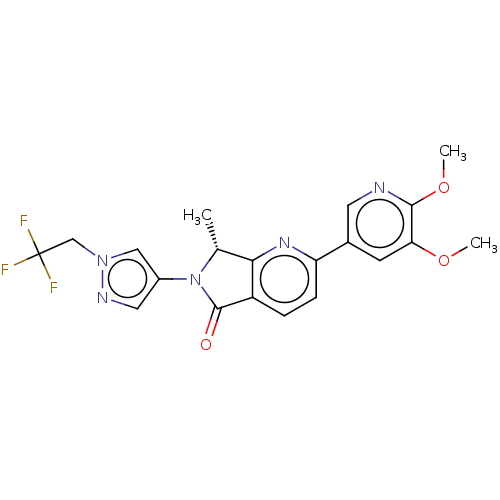
(Homo sapiens (Human)) | BDBM50274538
(CHEMBL4126773)Show SMILES COc1cc(cnc1OC)-c1ccc2C(=O)N([C@H](C)c2n1)c1cnn(CC(F)(F)F)c1 |r| Show InChI InChI=1S/C20H18F3N5O3/c1-11-17-14(19(29)28(11)13-8-25-27(9-13)10-20(21,22)23)4-5-15(26-17)12-6-16(30-2)18(31-3)24-7-12/h4-9,11H,10H2,1-3H3/t11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method |
J Med Chem 61: 5245-5256 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00085
BindingDB Entry DOI: 10.7270/Q2V98BK8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data