 Found 75 hits with Last Name = 'nchinda' and Initial = 'at'
Found 75 hits with Last Name = 'nchinda' and Initial = 'at' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50017125
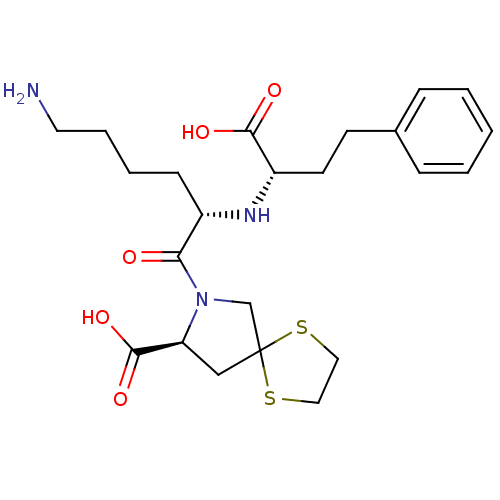
(1-[6-Amino-2-(1-carboxy-3-phenyl-propylamino)-hexa...)Show SMILES NCCCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1CC2(C[C@H]1C(O)=O)SCCS2 Show InChI InChI=1S/C23H33N3O5S2/c24-11-5-4-8-17(25-18(21(28)29)10-9-16-6-2-1-3-7-16)20(27)26-15-23(32-12-13-33-23)14-19(26)22(30)31/h1-3,6-7,17-19,25H,4-5,8-15,24H2,(H,28,29)(H,30,31)/t17-,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of testis ACE C domain |
Bioorg Med Chem Lett 16: 4616-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.004
BindingDB Entry DOI: 10.7270/Q2KP81SJ |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50017125

(1-[6-Amino-2-(1-carboxy-3-phenyl-propylamino)-hexa...)Show SMILES NCCCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1CC2(C[C@H]1C(O)=O)SCCS2 Show InChI InChI=1S/C23H33N3O5S2/c24-11-5-4-8-17(25-18(21(28)29)10-9-16-6-2-1-3-7-16)20(27)26-15-23(32-12-13-33-23)14-19(26)22(30)31/h1-3,6-7,17-19,25H,4-5,8-15,24H2,(H,28,29)(H,30,31)/t17-,18-,19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of rabbit testis recombinant ACE C domain |
Bioorg Med Chem Lett 16: 4612-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.003
BindingDB Entry DOI: 10.7270/Q2B56JC8 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50017125

(1-[6-Amino-2-(1-carboxy-3-phenyl-propylamino)-hexa...)Show SMILES NCCCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1CC2(C[C@H]1C(O)=O)SCCS2 Show InChI InChI=1S/C23H33N3O5S2/c24-11-5-4-8-17(25-18(21(28)29)10-9-16-6-2-1-3-7-16)20(27)26-15-23(32-12-13-33-23)14-19(26)22(30)31/h1-3,6-7,17-19,25H,4-5,8-15,24H2,(H,28,29)(H,30,31)/t17-,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 132 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of somatic ACE N domain |
Bioorg Med Chem Lett 16: 4616-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.004
BindingDB Entry DOI: 10.7270/Q2KP81SJ |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50017125

(1-[6-Amino-2-(1-carboxy-3-phenyl-propylamino)-hexa...)Show SMILES NCCCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1CC2(C[C@H]1C(O)=O)SCCS2 Show InChI InChI=1S/C23H33N3O5S2/c24-11-5-4-8-17(25-18(21(28)29)10-9-16-6-2-1-3-7-16)20(27)26-15-23(32-12-13-33-23)14-19(26)22(30)31/h1-3,6-7,17-19,25H,4-5,8-15,24H2,(H,28,29)(H,30,31)/t17-,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 132 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of recombinant ACE N domain |
Bioorg Med Chem Lett 16: 4612-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.003
BindingDB Entry DOI: 10.7270/Q2B56JC8 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50189517

((S)-2-((S)-5-benzamido-4-oxo-6-phenylhexanamido)-3...)Show SMILES OC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CCC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccccc1 Show InChI InChI=1S/C30H29N3O5/c34-27(25(17-20-9-3-1-4-10-20)33-29(36)21-11-5-2-6-12-21)15-16-28(35)32-26(30(37)38)18-22-19-31-24-14-8-7-13-23(22)24/h1-14,19,25-26,31H,15-18H2,(H,32,35)(H,33,36)(H,37,38)/t25-,26-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of rabbit testis recombinant ACE C domain |
Bioorg Med Chem Lett 16: 4612-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.003
BindingDB Entry DOI: 10.7270/Q2B56JC8 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50027344
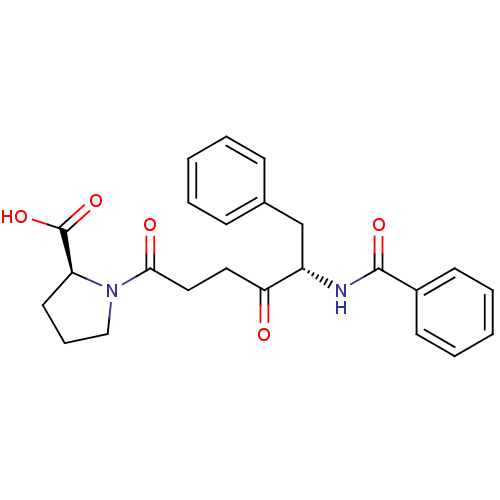
((S)-1-((S)-5-benzamido-4-oxo-6-phenylhexanoyl)pyrr...)Show SMILES OC(=O)[C@@H]1CCCN1C(=O)CCC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccccc1 Show InChI InChI=1S/C24H26N2O5/c27-21(13-14-22(28)26-15-7-12-20(26)24(30)31)19(16-17-8-3-1-4-9-17)25-23(29)18-10-5-2-6-11-18/h1-6,8-11,19-20H,7,12-16H2,(H,25,29)(H,30,31)/t19-,20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of rabbit testis recombinant ACE C domain |
Bioorg Med Chem Lett 16: 4612-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.003
BindingDB Entry DOI: 10.7270/Q2B56JC8 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50189452
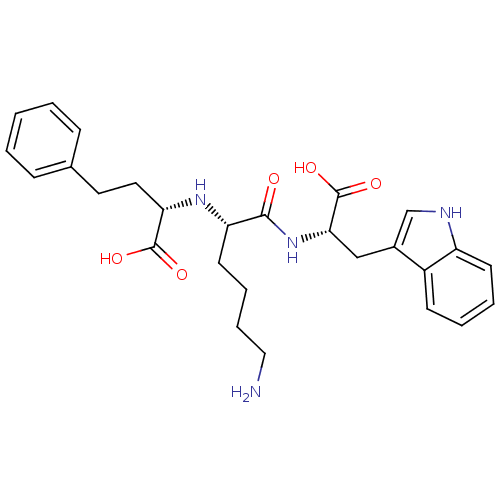
((S)-2-((S)-6-amino-1-((S)-1-carboxy-2-(1H-indol-3-...)Show SMILES NCCCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C27H34N4O5/c28-15-7-6-12-22(30-23(26(33)34)14-13-18-8-2-1-3-9-18)25(32)31-24(27(35)36)16-19-17-29-21-11-5-4-10-20(19)21/h1-5,8-11,17,22-24,29-30H,6-7,12-16,28H2,(H,31,32)(H,33,34)(H,35,36)/t22-,23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of testis ACE C domain |
Bioorg Med Chem Lett 16: 4616-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.004
BindingDB Entry DOI: 10.7270/Q2KP81SJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50189520
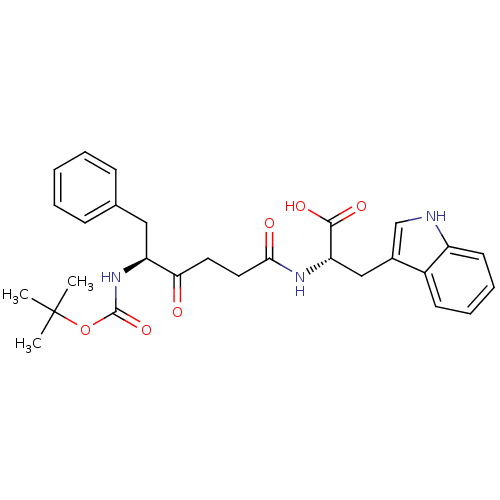
((S)-2-((S)-5-(tert-butoxycarbonyl)-4-oxo-6-phenylh...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)C(=O)CCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C28H33N3O6/c1-28(2,3)37-27(36)31-22(15-18-9-5-4-6-10-18)24(32)13-14-25(33)30-23(26(34)35)16-19-17-29-21-12-8-7-11-20(19)21/h4-12,17,22-23,29H,13-16H2,1-3H3,(H,30,33)(H,31,36)(H,34,35)/t22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of recombinant ACE N domain |
Bioorg Med Chem Lett 16: 4612-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.003
BindingDB Entry DOI: 10.7270/Q2B56JC8 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50189519
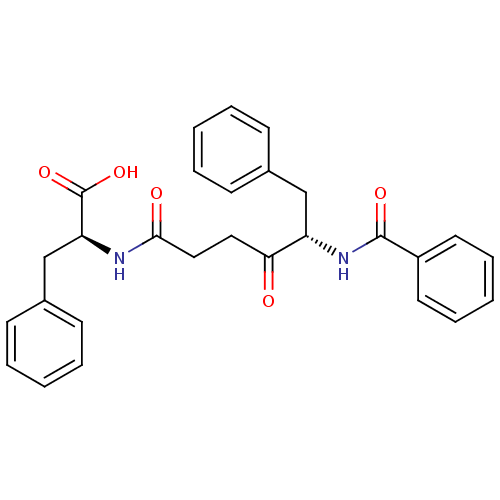
((S)-2-((S)-5-benzamido-4-oxo-6-phenylhexanamido)-3...)Show SMILES OC(=O)[C@H](Cc1ccccc1)NC(=O)CCC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccccc1 Show InChI InChI=1S/C28H28N2O5/c31-25(16-17-26(32)29-24(28(34)35)19-21-12-6-2-7-13-21)23(18-20-10-4-1-5-11-20)30-27(33)22-14-8-3-9-15-22/h1-15,23-24H,16-19H2,(H,29,32)(H,30,33)(H,34,35)/t23-,24-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of rabbit testis recombinant ACE C domain |
Bioorg Med Chem Lett 16: 4612-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.003
BindingDB Entry DOI: 10.7270/Q2B56JC8 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50189454

((R)-2-((S)-6-amino-1-((S)-1-carboxy-2-(1H-indol-3-...)Show SMILES NCCCC[C@H](N[C@H](CCc1ccccc1)C(O)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C27H34N4O5/c28-15-7-6-12-22(30-23(26(33)34)14-13-18-8-2-1-3-9-18)25(32)31-24(27(35)36)16-19-17-29-21-11-5-4-10-20(19)21/h1-5,8-11,17,22-24,29-30H,6-7,12-16,28H2,(H,31,32)(H,33,34)(H,35,36)/t22-,23+,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of testis ACE C domain |
Bioorg Med Chem Lett 16: 4616-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.004
BindingDB Entry DOI: 10.7270/Q2KP81SJ |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50189518
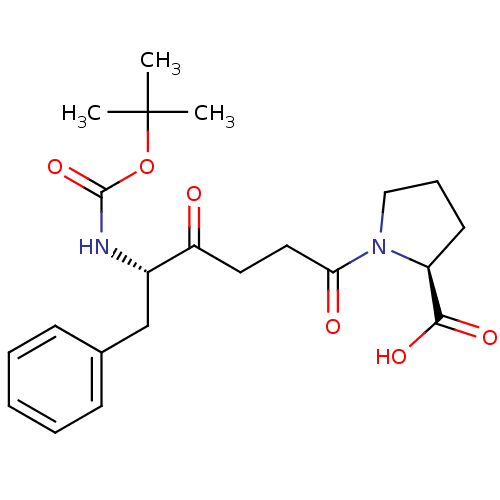
((S)-1-((S)-5-(tert-butoxycarbonyl)-4-oxo-6-phenylh...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)C(=O)CCC(=O)N1CCC[C@H]1C(O)=O Show InChI InChI=1S/C22H30N2O6/c1-22(2,3)30-21(29)23-16(14-15-8-5-4-6-9-15)18(25)11-12-19(26)24-13-7-10-17(24)20(27)28/h4-6,8-9,16-17H,7,10-14H2,1-3H3,(H,23,29)(H,27,28)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of recombinant ACE N domain |
Bioorg Med Chem Lett 16: 4612-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.003
BindingDB Entry DOI: 10.7270/Q2B56JC8 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50189521
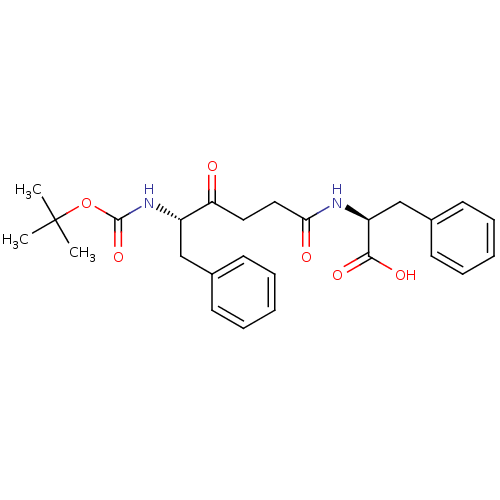
((S)-2-((S)-5-(tert-butoxycarbonyl)-4-oxo-6-phenylh...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)C(=O)CCC(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C26H32N2O6/c1-26(2,3)34-25(33)28-20(16-18-10-6-4-7-11-18)22(29)14-15-23(30)27-21(24(31)32)17-19-12-8-5-9-13-19/h4-13,20-21H,14-17H2,1-3H3,(H,27,30)(H,28,33)(H,31,32)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of recombinant ACE N domain |
Bioorg Med Chem Lett 16: 4612-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.003
BindingDB Entry DOI: 10.7270/Q2B56JC8 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50027344

((S)-1-((S)-5-benzamido-4-oxo-6-phenylhexanoyl)pyrr...)Show SMILES OC(=O)[C@@H]1CCCN1C(=O)CCC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccccc1 Show InChI InChI=1S/C24H26N2O5/c27-21(13-14-22(28)26-15-7-12-20(26)24(30)31)19(16-17-8-3-1-4-9-17)25-23(29)18-10-5-2-6-11-18/h1-6,8-11,19-20H,7,12-16H2,(H,25,29)(H,30,31)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of recombinant ACE N domain |
Bioorg Med Chem Lett 16: 4612-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.003
BindingDB Entry DOI: 10.7270/Q2B56JC8 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50189519

((S)-2-((S)-5-benzamido-4-oxo-6-phenylhexanamido)-3...)Show SMILES OC(=O)[C@H](Cc1ccccc1)NC(=O)CCC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccccc1 Show InChI InChI=1S/C28H28N2O5/c31-25(16-17-26(32)29-24(28(34)35)19-21-12-6-2-7-13-21)23(18-20-10-4-1-5-11-20)30-27(33)22-14-8-3-9-15-22/h1-15,23-24H,16-19H2,(H,29,32)(H,30,33)(H,34,35)/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of recombinant ACE N domain |
Bioorg Med Chem Lett 16: 4612-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.003
BindingDB Entry DOI: 10.7270/Q2B56JC8 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50189518

((S)-1-((S)-5-(tert-butoxycarbonyl)-4-oxo-6-phenylh...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)C(=O)CCC(=O)N1CCC[C@H]1C(O)=O Show InChI InChI=1S/C22H30N2O6/c1-22(2,3)30-21(29)23-16(14-15-8-5-4-6-9-15)18(25)11-12-19(26)24-13-7-10-17(24)20(27)28/h4-6,8-9,16-17H,7,10-14H2,1-3H3,(H,23,29)(H,27,28)/t16-,17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.44E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of rabbit testis recombinant ACE C domain |
Bioorg Med Chem Lett 16: 4612-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.003
BindingDB Entry DOI: 10.7270/Q2B56JC8 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50189517

((S)-2-((S)-5-benzamido-4-oxo-6-phenylhexanamido)-3...)Show SMILES OC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CCC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccccc1 Show InChI InChI=1S/C30H29N3O5/c34-27(25(17-20-9-3-1-4-10-20)33-29(36)21-11-5-2-6-12-21)15-16-28(35)32-26(30(37)38)18-22-19-31-24-14-8-7-13-23(22)24/h1-14,19,25-26,31H,15-18H2,(H,32,35)(H,33,36)(H,37,38)/t25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.96E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of recombinant ACE N domain |
Bioorg Med Chem Lett 16: 4612-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.003
BindingDB Entry DOI: 10.7270/Q2B56JC8 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50189521

((S)-2-((S)-5-(tert-butoxycarbonyl)-4-oxo-6-phenylh...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)C(=O)CCC(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C26H32N2O6/c1-26(2,3)34-25(33)28-20(16-18-10-6-4-7-11-18)22(29)14-15-23(30)27-21(24(31)32)17-19-12-8-5-9-13-19/h4-13,20-21H,14-17H2,1-3H3,(H,27,30)(H,28,33)(H,31,32)/t20-,21-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.13E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of rabbit testis recombinant ACE C domain |
Bioorg Med Chem Lett 16: 4612-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.003
BindingDB Entry DOI: 10.7270/Q2B56JC8 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50189520

((S)-2-((S)-5-(tert-butoxycarbonyl)-4-oxo-6-phenylh...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)C(=O)CCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C28H33N3O6/c1-28(2,3)37-27(36)31-22(15-18-9-5-4-6-10-18)24(32)13-14-25(33)30-23(26(34)35)16-19-17-29-21-12-8-7-11-20(19)21/h4-12,17,22-23,29H,13-16H2,1-3H3,(H,30,33)(H,31,36)(H,34,35)/t22-,23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 3.81E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of rabbit testis recombinant ACE C domain |
Bioorg Med Chem Lett 16: 4612-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.003
BindingDB Entry DOI: 10.7270/Q2B56JC8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50357427
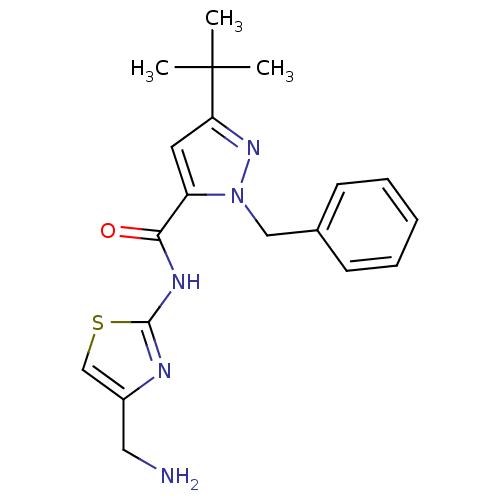
(CHEMBL1917511)Show SMILES CC(C)(C)c1cc(C(=O)Nc2nc(CN)cs2)n(Cc2ccccc2)n1 Show InChI InChI=1S/C19H23N5OS/c1-19(2,3)16-9-15(17(25)22-18-21-14(10-20)12-26-18)24(23-16)11-13-7-5-4-6-8-13/h4-9,12H,10-11,20H2,1-3H3,(H,21,22,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6-mediated dextromethorphan O-demethylation in human liver microsomes by LCMS analysis |
J Med Chem 54: 7713-9 (2011)
Article DOI: 10.1021/jm201108k
BindingDB Entry DOI: 10.7270/Q20865Q4 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50554407
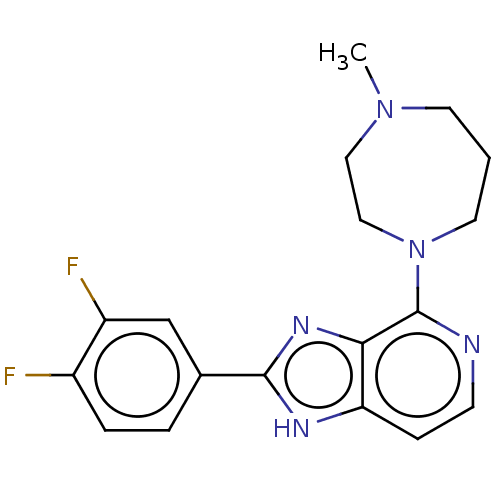
(CHEMBL4746185)Show SMILES CN1CCCN(CC1)c1nccc2[nH]c(nc12)-c1ccc(F)c(F)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG by patch clamp method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01411
BindingDB Entry DOI: 10.7270/Q2TB1BKN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50554417

(CHEMBL4758157)Show SMILES CN1CCCN(CC1)c1nccc2[nH]c(nc12)-c1cc(F)cc(F)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG by patch clamp method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01411
BindingDB Entry DOI: 10.7270/Q2TB1BKN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50503392
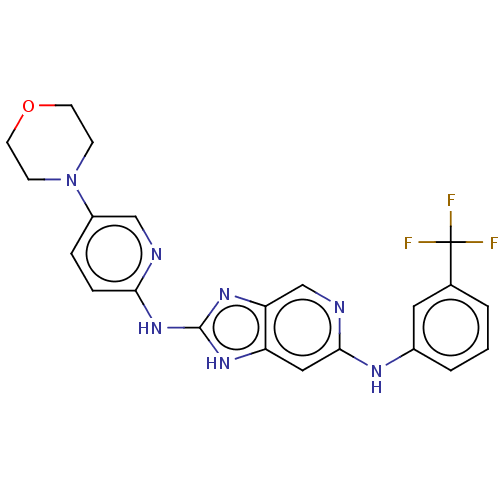
(CHEMBL4545990)Show SMILES FC(F)(F)c1cccc(Nc2cc3[nH]c(Nc4ccc(cn4)N4CCOCC4)nc3cn2)c1 Show InChI InChI=1S/C22H20F3N7O/c23-22(24,25)14-2-1-3-15(10-14)28-20-11-17-18(13-27-20)30-21(29-17)31-19-5-4-16(12-26-19)32-6-8-33-9-7-32/h1-5,10-13H,6-9H2,(H,27,28)(H2,26,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp electrophysiology |
J Med Chem 61: 9371-9385 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01333
BindingDB Entry DOI: 10.7270/Q2222Z1Q |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50357427

(CHEMBL1917511)Show SMILES CC(C)(C)c1cc(C(=O)Nc2nc(CN)cs2)n(Cc2ccccc2)n1 Show InChI InChI=1S/C19H23N5OS/c1-19(2,3)16-9-15(17(25)22-18-21-14(10-20)12-26-18)24(23-16)11-13-7-5-4-6-8-13/h4-9,12H,10-11,20H2,1-3H3,(H,21,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2-mediated phencetin o-de-ethylation in human liver microsomes by LCMS analysis |
J Med Chem 54: 7713-9 (2011)
Article DOI: 10.1021/jm201108k
BindingDB Entry DOI: 10.7270/Q20865Q4 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50496040
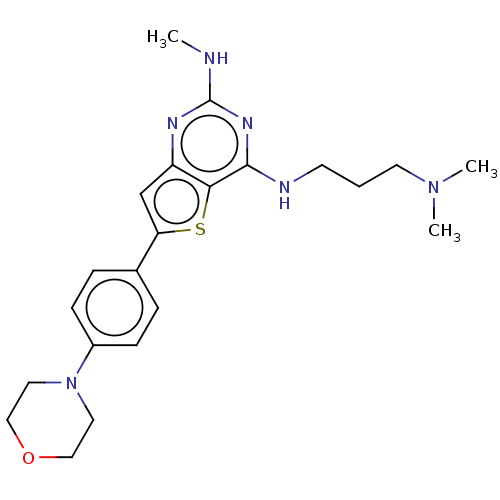
(CHEMBL3120287)Show SMILES CNc1nc(NCCCN(C)C)c2sc(cc2n1)-c1ccc(cc1)N1CCOCC1 Show InChI InChI=1S/C22H30N6OS/c1-23-22-25-18-15-19(30-20(18)21(26-22)24-9-4-10-27(2)3)16-5-7-17(8-6-16)28-11-13-29-14-12-28/h5-8,15H,4,9-14H2,1-3H3,(H2,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Binding affinity to full length human ERG expressed in CHO cells after 6 to 7 mins by IonWorks assay |
J Med Chem 57: 1014-22 (2014)
Article DOI: 10.1021/jm401760c
BindingDB Entry DOI: 10.7270/Q2NG4TMV |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50558661
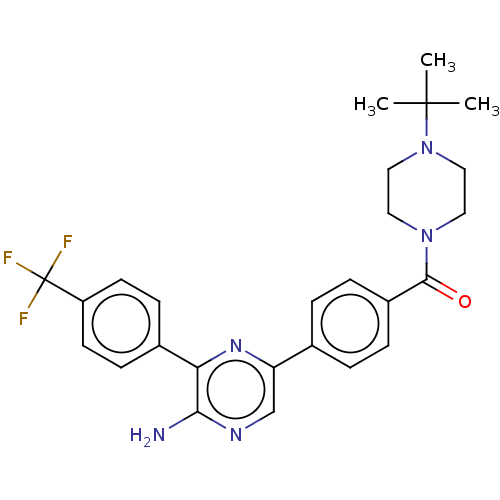
(CHEMBL4763231)Show SMILES CC(C)(C)N1CCN(CC1)C(=O)c1ccc(cc1)-c1cnc(N)c(n1)-c1ccc(cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG by IonWorks patch clamp electrophysiology method |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M61PZG |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50496036
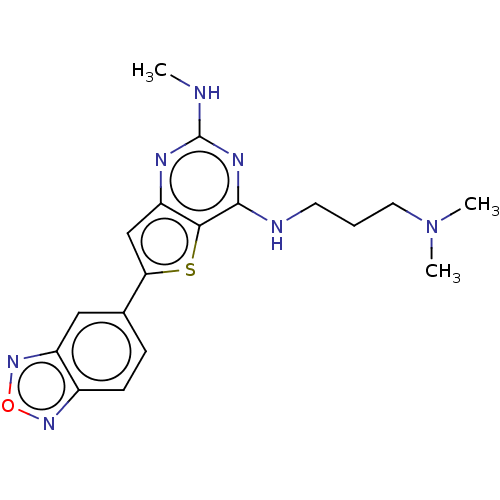
(CHEMBL3120293)Show InChI InChI=1S/C18H21N7OS/c1-19-18-21-14-10-15(11-5-6-12-13(9-11)24-26-23-12)27-16(14)17(22-18)20-7-4-8-25(2)3/h5-6,9-10H,4,7-8H2,1-3H3,(H2,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Binding affinity to full length human ERG expressed in CHO cells after 6 to 7 mins by IonWorks assay |
J Med Chem 57: 1014-22 (2014)
Article DOI: 10.1021/jm401760c
BindingDB Entry DOI: 10.7270/Q2NG4TMV |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50400228
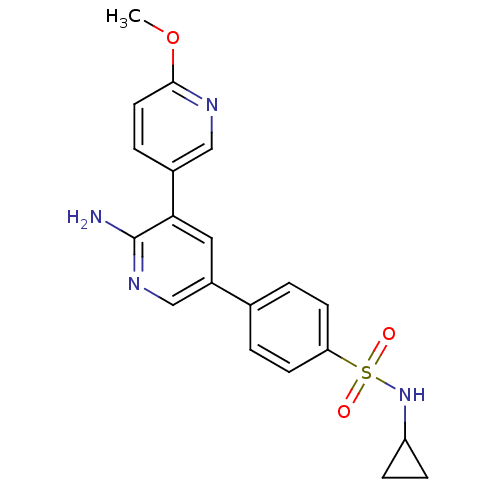
(CHEMBL2181495)Show SMILES COc1ccc(cn1)-c1cc(cnc1N)-c1ccc(cc1)S(=O)(=O)NC1CC1 Show InChI InChI=1S/C20H20N4O3S/c1-27-19-9-4-14(11-22-19)18-10-15(12-23-20(18)21)13-2-7-17(8-3-13)28(25,26)24-16-5-6-16/h2-4,7-12,16,24H,5-6H2,1H3,(H2,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in chinese hamster lung cells by IonWorks patch clamp electrophysiology assay |
J Med Chem 55: 11022-30 (2012)
Article DOI: 10.1021/jm301476b
BindingDB Entry DOI: 10.7270/Q2F76DQQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50122019
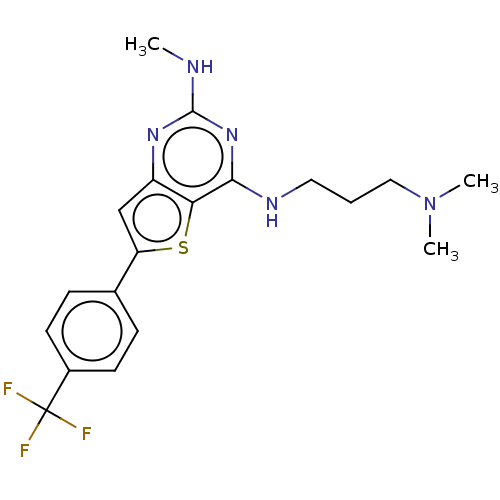
(CHEMBL3120274)Show SMILES CNc1nc(NCCCN(C)C)c2sc(cc2n1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C19H22F3N5S/c1-23-18-25-14-11-15(12-5-7-13(8-6-12)19(20,21)22)28-16(14)17(26-18)24-9-4-10-27(2)3/h5-8,11H,4,9-10H2,1-3H3,(H2,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Binding affinity to full length human ERG expressed in CHO cells after 6 to 7 mins by IonWorks assay |
J Med Chem 57: 1014-22 (2014)
Article DOI: 10.1021/jm401760c
BindingDB Entry DOI: 10.7270/Q2NG4TMV |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50496037
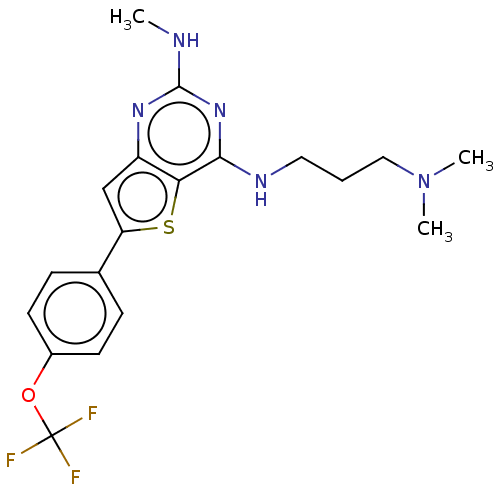
(CHEMBL3120280)Show SMILES CNc1nc(NCCCN(C)C)c2sc(cc2n1)-c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C19H22F3N5OS/c1-23-18-25-14-11-15(12-5-7-13(8-6-12)28-19(20,21)22)29-16(14)17(26-18)24-9-4-10-27(2)3/h5-8,11H,4,9-10H2,1-3H3,(H2,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Binding affinity to full length human ERG expressed in CHO cells after 6 to 7 mins by IonWorks assay |
J Med Chem 57: 1014-22 (2014)
Article DOI: 10.1021/jm401760c
BindingDB Entry DOI: 10.7270/Q2NG4TMV |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50385911
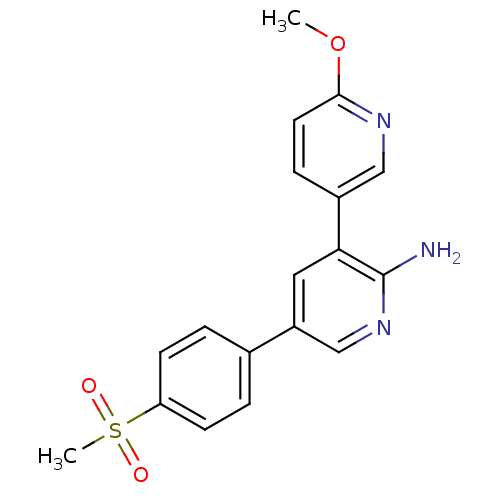
(CHEMBL531475 | TCMDC-133351)Show SMILES COc1ccc(cn1)-c1cc(cnc1N)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C18H17N3O3S/c1-24-17-8-5-13(10-20-17)16-9-14(11-21-18(16)19)12-3-6-15(7-4-12)25(2,22)23/h3-11H,1-2H3,(H2,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHO cells by IonWorks assay |
J Med Chem 55: 3479-87 (2012)
Article DOI: 10.1021/jm3001373
BindingDB Entry DOI: 10.7270/Q2V125T9 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50554415
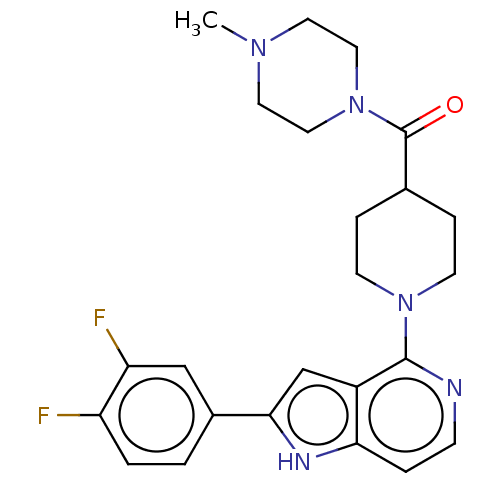
(CHEMBL4757759)Show SMILES CN1CCN(CC1)C(=O)C1CCN(CC1)c1nccc2[nH]c(cc12)-c1ccc(F)c(F)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG by patch clamp method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01411
BindingDB Entry DOI: 10.7270/Q2TB1BKN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50400227

(CHEMBL2181489)Show SMILES Nc1ncc(cc1-c1ccc(cc1)C(F)(F)F)-c1ccc(cc1)C(=O)N1CCOCC1 Show InChI InChI=1S/C23H20F3N3O2/c24-23(25,26)19-7-5-16(6-8-19)20-13-18(14-28-21(20)27)15-1-3-17(4-2-15)22(30)29-9-11-31-12-10-29/h1-8,13-14H,9-12H2,(H2,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in chinese hamster lung cells by IonWorks patch clamp electrophysiology assay |
J Med Chem 55: 11022-30 (2012)
Article DOI: 10.1021/jm301476b
BindingDB Entry DOI: 10.7270/Q2F76DQQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50554412
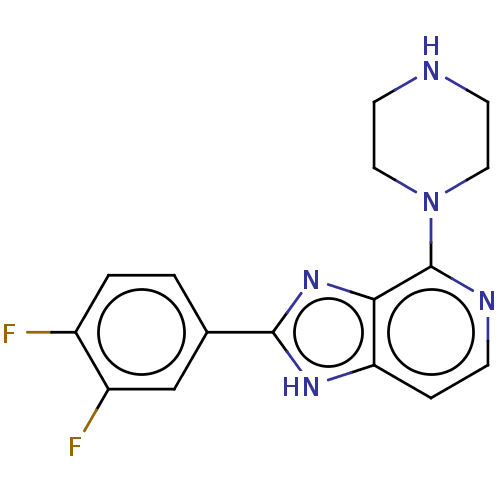
(CHEMBL4743530) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG by patch clamp method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01411
BindingDB Entry DOI: 10.7270/Q2TB1BKN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50357427

(CHEMBL1917511)Show SMILES CC(C)(C)c1cc(C(=O)Nc2nc(CN)cs2)n(Cc2ccccc2)n1 Show InChI InChI=1S/C19H23N5OS/c1-19(2,3)16-9-15(17(25)22-18-21-14(10-20)12-26-18)24(23-16)11-13-7-5-4-6-8-13/h4-9,12H,10-11,20H2,1-3H3,(H,21,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in Chinese hamster CHL cells by perforated patch clamp assay |
J Med Chem 54: 7713-9 (2011)
Article DOI: 10.1021/jm201108k
BindingDB Entry DOI: 10.7270/Q20865Q4 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50496198
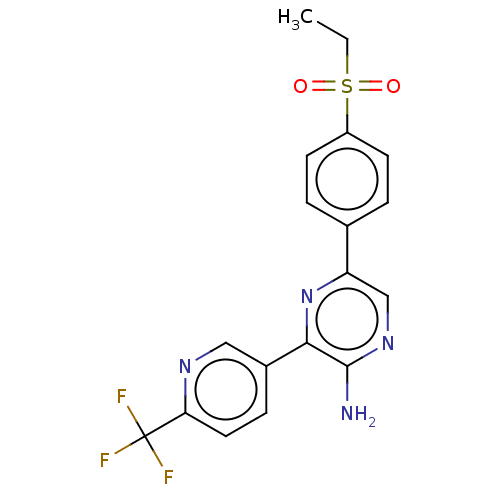
(CHEMBL3124985)Show SMILES CCS(=O)(=O)c1ccc(cc1)-c1cnc(N)c(n1)-c1ccc(nc1)C(F)(F)F Show InChI InChI=1S/C18H15F3N4O2S/c1-2-28(26,27)13-6-3-11(4-7-13)14-10-24-17(22)16(25-14)12-5-8-15(23-9-12)18(19,20)21/h3-10H,2H2,1H3,(H2,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp electrophysiology |
J Med Chem 56: 8860-71 (2013)
Article DOI: 10.1021/jm401278d
BindingDB Entry DOI: 10.7270/Q2GT5R5G |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50496197
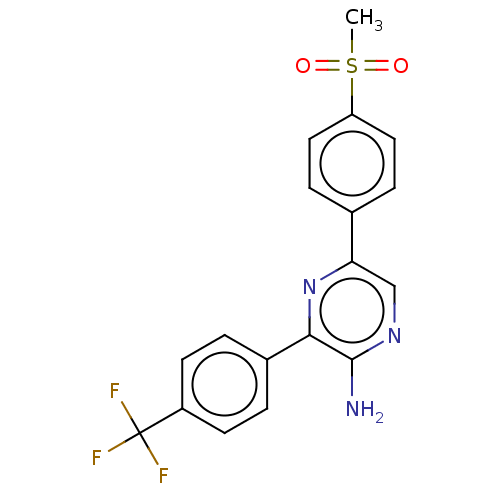
(CHEMBL3124981)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cnc(N)c(n1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C18H14F3N3O2S/c1-27(25,26)14-8-4-11(5-9-14)15-10-23-17(22)16(24-15)12-2-6-13(7-3-12)18(19,20)21/h2-10H,1H3,(H2,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp electrophysiology |
J Med Chem 56: 8860-71 (2013)
Article DOI: 10.1021/jm401278d
BindingDB Entry DOI: 10.7270/Q2GT5R5G |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50554416
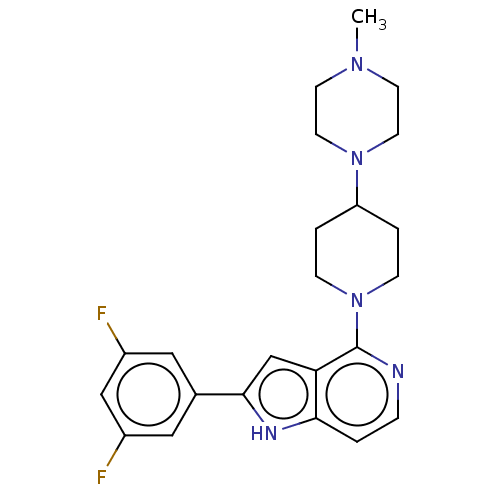
(CHEMBL4778630)Show SMILES CN1CCN(CC1)C1CCN(CC1)c1nccc2[nH]c(cc12)-c1cc(F)cc(F)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG by patch clamp method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01411
BindingDB Entry DOI: 10.7270/Q2TB1BKN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50385912
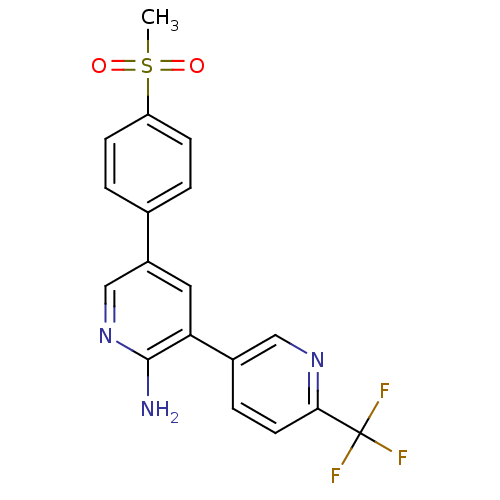
(CHEMBL2041980)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cnc(N)c(c1)-c1ccc(nc1)C(F)(F)F Show InChI InChI=1S/C18H14F3N3O2S/c1-27(25,26)14-5-2-11(3-6-14)13-8-15(17(22)24-10-13)12-4-7-16(23-9-12)18(19,20)21/h2-10H,1H3,(H2,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHO cells by IonWorks assay |
J Med Chem 55: 3479-87 (2012)
Article DOI: 10.1021/jm3001373
BindingDB Entry DOI: 10.7270/Q2V125T9 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50554410
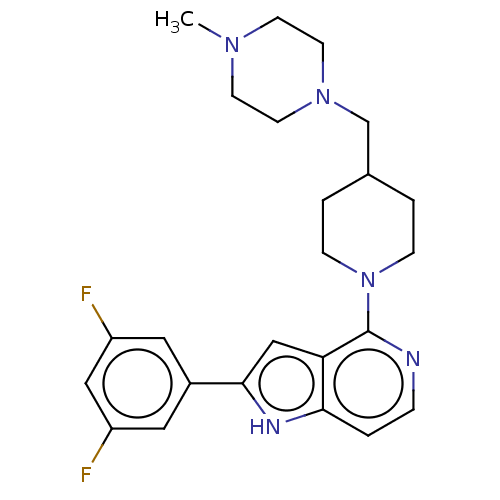
(CHEMBL4789774)Show SMILES CN1CCN(CC2CCN(CC2)c2nccc3[nH]c(cc23)-c2cc(F)cc(F)c2)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG by patch clamp method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01411
BindingDB Entry DOI: 10.7270/Q2TB1BKN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50558662

(CHEMBL4763365)Show SMILES CN1CCCN(CC1)C(=O)c1ccc(cc1)-c1cnc(N)c(n1)-c1ccc(cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG by IonWorks patch clamp electrophysiology method |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M61PZG |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50503394
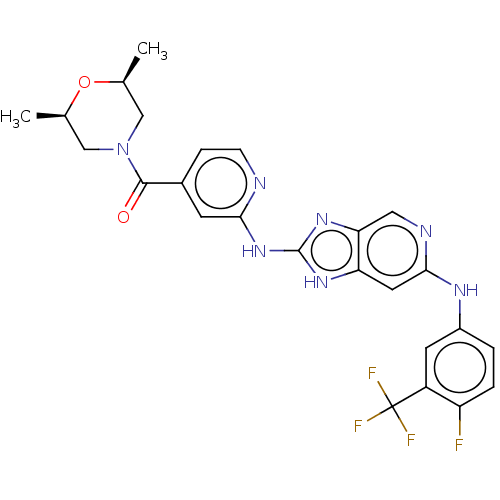
(CHEMBL4447741)Show SMILES C[C@H]1CN(C[C@@H](C)O1)C(=O)c1ccnc(Nc2nc3cnc(Nc4ccc(F)c(c4)C(F)(F)F)cc3[nH]2)c1 |r| Show InChI InChI=1S/C25H23F4N7O2/c1-13-11-36(12-14(2)38-13)23(37)15-5-6-30-21(7-15)35-24-33-19-9-22(31-10-20(19)34-24)32-16-3-4-18(26)17(8-16)25(27,28)29/h3-10,13-14H,11-12H2,1-2H3,(H,31,32)(H2,30,33,34,35)/t13-,14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp electrophysiology |
J Med Chem 61: 9371-9385 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01333
BindingDB Entry DOI: 10.7270/Q2222Z1Q |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50400226

(CHEMBL2181298)Show SMILES Nc1ncc(cc1-c1ccc(cc1)C(F)(F)F)-c1ccc(cc1)C(=O)N1CCNCC1 Show InChI InChI=1S/C23H21F3N4O/c24-23(25,26)19-7-5-16(6-8-19)20-13-18(14-29-21(20)27)15-1-3-17(4-2-15)22(31)30-11-9-28-10-12-30/h1-8,13-14,28H,9-12H2,(H2,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in chinese hamster lung cells by IonWorks patch clamp electrophysiology assay |
J Med Chem 55: 11022-30 (2012)
Article DOI: 10.1021/jm301476b
BindingDB Entry DOI: 10.7270/Q2F76DQQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50496039
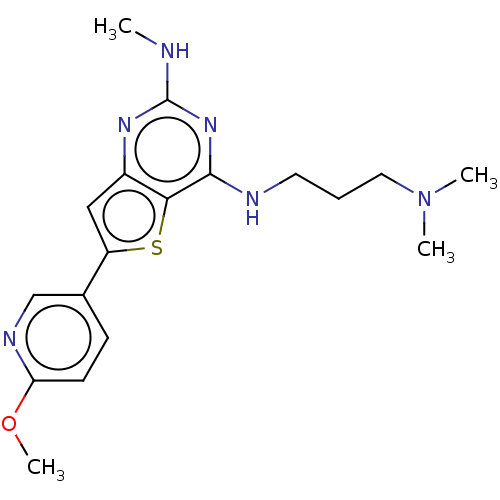
(CHEMBL3120281)Show InChI InChI=1S/C18H24N6OS/c1-19-18-22-13-10-14(12-6-7-15(25-4)21-11-12)26-16(13)17(23-18)20-8-5-9-24(2)3/h6-7,10-11H,5,8-9H2,1-4H3,(H2,19,20,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Binding affinity to full length human ERG expressed in CHO cells after 6 to 7 mins by IonWorks assay |
J Med Chem 57: 1014-22 (2014)
Article DOI: 10.1021/jm401760c
BindingDB Entry DOI: 10.7270/Q2NG4TMV |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50496202
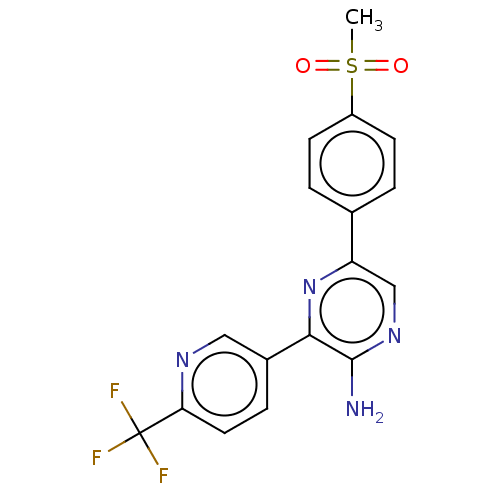
(CHEMBL3124980)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cnc(N)c(n1)-c1ccc(nc1)C(F)(F)F Show InChI InChI=1S/C17H13F3N4O2S/c1-27(25,26)12-5-2-10(3-6-12)13-9-23-16(21)15(24-13)11-4-7-14(22-8-11)17(18,19)20/h2-9H,1H3,(H2,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp electrophysiology |
J Med Chem 56: 8860-71 (2013)
Article DOI: 10.1021/jm401278d
BindingDB Entry DOI: 10.7270/Q2GT5R5G |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50400225

(CHEMBL2181490)Show SMILES CN1CCN(CC1)C(=O)c1ccc(cc1)-c1cnc(N)c(c1)-c1ccc(nc1)C(F)(F)F Show InChI InChI=1S/C23H22F3N5O/c1-30-8-10-31(11-9-30)22(32)16-4-2-15(3-5-16)18-12-19(21(27)29-14-18)17-6-7-20(28-13-17)23(24,25)26/h2-7,12-14H,8-11H2,1H3,(H2,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in chinese hamster lung cells by IonWorks patch clamp electrophysiology assay |
J Med Chem 55: 11022-30 (2012)
Article DOI: 10.1021/jm301476b
BindingDB Entry DOI: 10.7270/Q2F76DQQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50496038

(CHEMBL3120265)Show InChI InChI=1S/C18H23N5S/c1-19-18-21-14-12-15(13-8-5-4-6-9-13)24-16(14)17(22-18)20-10-7-11-23(2)3/h4-6,8-9,12H,7,10-11H2,1-3H3,(H2,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Binding affinity to full length human ERG expressed in CHO cells after 6 to 7 mins by IonWorks assay |
J Med Chem 57: 1014-22 (2014)
Article DOI: 10.1021/jm401760c
BindingDB Entry DOI: 10.7270/Q2NG4TMV |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50400224

(CHEMBL2181492)Show SMILES COc1ccc(cn1)-c1cc(cnc1N)-c1ccc(cc1)S(=O)(=O)N(C)C Show InChI InChI=1S/C19H20N4O3S/c1-23(2)27(24,25)16-7-4-13(5-8-16)15-10-17(19(20)22-12-15)14-6-9-18(26-3)21-11-14/h4-12H,1-3H3,(H2,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in chinese hamster lung cells by IonWorks patch clamp electrophysiology assay |
J Med Chem 55: 11022-30 (2012)
Article DOI: 10.1021/jm301476b
BindingDB Entry DOI: 10.7270/Q2F76DQQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50558663

(CHEMBL4798032)Show SMILES Nc1ncc(nc1-c1ccc(cc1)C(F)(F)F)-c1ccc(cc1)C(=O)N1CCNCC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG by IonWorks patch clamp electrophysiology method |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M61PZG |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50558659

(CHEMBL4530341)Show SMILES NC1CCN(C1)C(=O)c1ccc(cc1)-c1cnc(N)c(n1)-c1ccc(cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG by IonWorks patch clamp electrophysiology method |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M61PZG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50357427

(CHEMBL1917511)Show SMILES CC(C)(C)c1cc(C(=O)Nc2nc(CN)cs2)n(Cc2ccccc2)n1 Show InChI InChI=1S/C19H23N5OS/c1-19(2,3)16-9-15(17(25)22-18-21-14(10-20)12-26-18)24(23-16)11-13-7-5-4-6-8-13/h4-9,12H,10-11,20H2,1-3H3,(H,21,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9-mediated Tolbutamide methlyhydroxylation in human liver microsomes by LCMS analysis |
J Med Chem 54: 7713-9 (2011)
Article DOI: 10.1021/jm201108k
BindingDB Entry DOI: 10.7270/Q20865Q4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data