 Found 36 hits with Last Name = 'sasaki' and Initial = 'at'
Found 36 hits with Last Name = 'sasaki' and Initial = 'at' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
1-phosphatidylinositol 3-phosphate 5-kinase
(Homo sapiens) | BDBM50511422
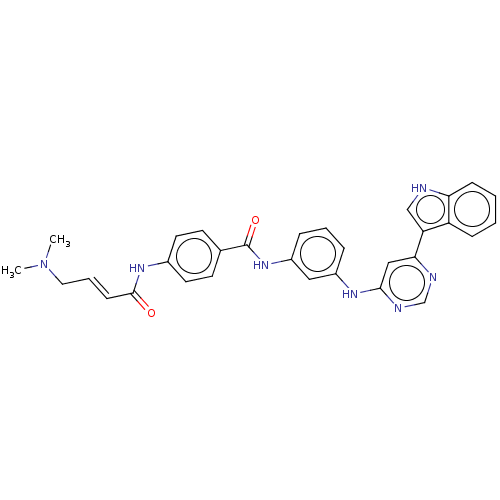
(CHEMBL4446338)Show SMILES CN(C)C\C=C\C(=O)Nc1ccc(cc1)C(=O)Nc1cccc(Nc2cc(ncn2)-c2c[nH]c3ccccc23)c1 Show InChI InChI=1S/C31H29N7O2/c1-38(2)16-6-11-30(39)36-22-14-12-21(13-15-22)31(40)37-24-8-5-7-23(17-24)35-29-18-28(33-20-34-29)26-19-32-27-10-4-3-9-25(26)27/h3-15,17-20,32H,16H2,1-2H3,(H,36,39)(H,37,40)(H,33,34,35)/b11-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged full length human PIKFYVE (1 to 2098 residues) using PI(3)P and Phosphatidylserine as substrate by ADP-Glo assay |
ACS Med Chem Lett 11: 346-352 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00402
BindingDB Entry DOI: 10.7270/Q2TM7FDP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha
(Homo sapiens) | BDBM50511422

(CHEMBL4446338)Show SMILES CN(C)C\C=C\C(=O)Nc1ccc(cc1)C(=O)Nc1cccc(Nc2cc(ncn2)-c2c[nH]c3ccccc23)c1 Show InChI InChI=1S/C31H29N7O2/c1-38(2)16-6-11-30(39)36-22-14-12-21(13-15-22)31(40)37-24-8-5-7-23(17-24)35-29-18-28(33-20-34-29)26-19-32-27-10-4-3-9-25(26)27/h3-15,17-20,32H,16H2,1-2H3,(H,36,39)(H,37,40)(H,33,34,35)/b11-6+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of PI5P4Kalpha (unknown origin) incubated for 15 mins in presence of ATP by ADP-Glo assay |
ACS Med Chem Lett 11: 346-352 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00402
BindingDB Entry DOI: 10.7270/Q2TM7FDP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha
(Homo sapiens) | BDBM60983

((E)-2-(3,4-dihydroxybenzoyl)-3-(4-hydroxy-3-iodo-5...)Show SMILES COc1cc(\C=C(/C#N)C(=O)c2ccc(O)c(O)c2)cc(I)c1O Show InChI InChI=1S/C17H12INO5/c1-24-15-6-9(5-12(18)17(15)23)4-11(8-19)16(22)10-2-3-13(20)14(21)7-10/h2-7,20-21,23H,1H3/b11-4+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of PI5P4Kalpha (unknown origin) incubated for 15 mins in presence of ATP by ADP-Glo assay |
ACS Med Chem Lett 11: 346-352 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00402
BindingDB Entry DOI: 10.7270/Q2TM7FDP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 gamma
(Homo sapiens (Human)) | BDBM50497347
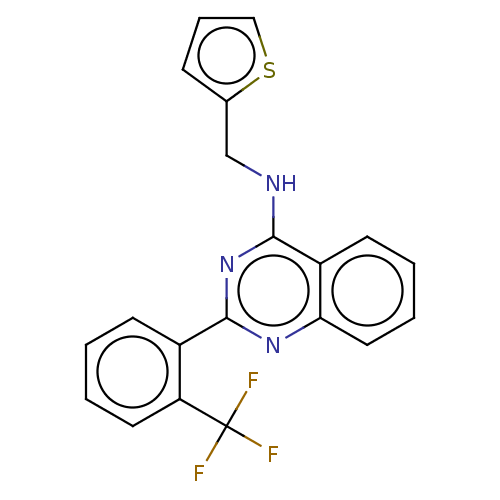
(CHEMBL1529478)Show InChI InChI=1S/C20H14F3N3S/c21-20(22,23)16-9-3-1-7-14(16)19-25-17-10-4-2-8-15(17)18(26-19)24-12-13-6-5-11-27-13/h1-11H,12H2,(H,24,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of PI5P4Kgamma (unknown origin) |
ACS Med Chem Lett 11: 346-352 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00402
BindingDB Entry DOI: 10.7270/Q2TM7FDP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha
(Homo sapiens) | BDBM50511420
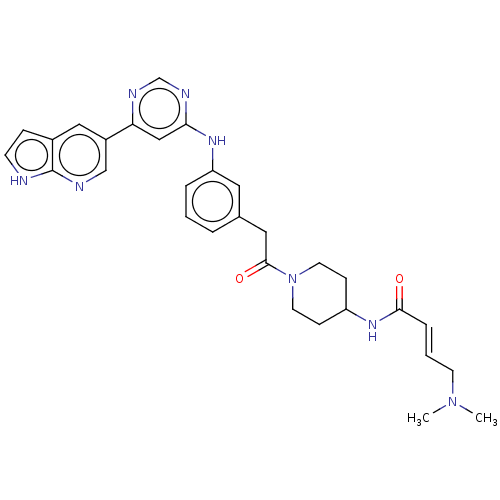
(CHEMBL4449493)Show SMILES CN(C)C\C=C\C(=O)NC1CCN(CC1)C(=O)Cc1cccc(Nc2cc(ncn2)-c2cnc3[nH]ccc3c2)c1 Show InChI InChI=1S/C30H34N8O2/c1-37(2)12-4-7-28(39)36-24-9-13-38(14-10-24)29(40)16-21-5-3-6-25(15-21)35-27-18-26(33-20-34-27)23-17-22-8-11-31-30(22)32-19-23/h3-8,11,15,17-20,24H,9-10,12-14,16H2,1-2H3,(H,31,32)(H,36,39)(H,33,34,35)/b7-4+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of PI5P4Kalpha (unknown origin) incubated for 15 mins in presence of ATP by ADP-Glo assay |
ACS Med Chem Lett 11: 346-352 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00402
BindingDB Entry DOI: 10.7270/Q2TM7FDP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha
(Homo sapiens) | BDBM50511414

(CHEMBL4472811)Show SMILES CN(C)C\C=C\C(=O)Nc1ccc(cc1)C(=O)Nc1cccc(Nc2cc(Nc3ccc(cc3)-c3ccccc3)ncn2)c1 Show InChI InChI=1S/C35H33N7O2/c1-42(2)21-7-12-34(43)40-29-19-15-27(16-20-29)35(44)41-31-11-6-10-30(22-31)39-33-23-32(36-24-37-33)38-28-17-13-26(14-18-28)25-8-4-3-5-9-25/h3-20,22-24H,21H2,1-2H3,(H,40,43)(H,41,44)(H2,36,37,38,39)/b12-7+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of PI5P4Kalpha (unknown origin) incubated for 15 mins in presence of ATP by ADP-Glo assay |
ACS Med Chem Lett 11: 346-352 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00402
BindingDB Entry DOI: 10.7270/Q2TM7FDP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha
(Homo sapiens) | BDBM50511415
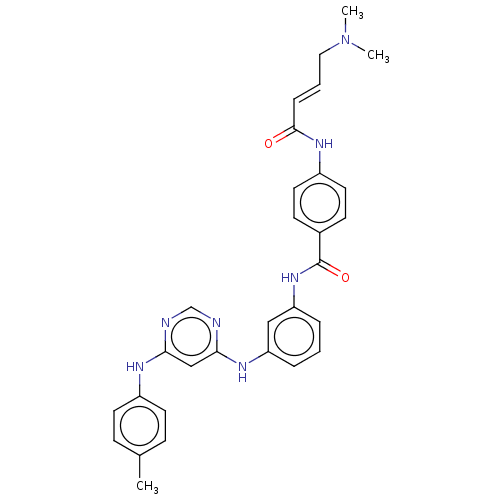
(CHEMBL4461694)Show SMILES CN(C)C\C=C\C(=O)Nc1ccc(cc1)C(=O)Nc1cccc(Nc2cc(Nc3ccc(C)cc3)ncn2)c1 Show InChI InChI=1S/C30H31N7O2/c1-21-9-13-23(14-10-21)33-27-19-28(32-20-31-27)34-25-6-4-7-26(18-25)36-30(39)22-11-15-24(16-12-22)35-29(38)8-5-17-37(2)3/h4-16,18-20H,17H2,1-3H3,(H,35,38)(H,36,39)(H2,31,32,33,34)/b8-5+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of PI5P4Kalpha (unknown origin) incubated for 15 mins in presence of ATP by ADP-Glo assay |
ACS Med Chem Lett 11: 346-352 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00402
BindingDB Entry DOI: 10.7270/Q2TM7FDP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha
(Homo sapiens) | BDBM50511421
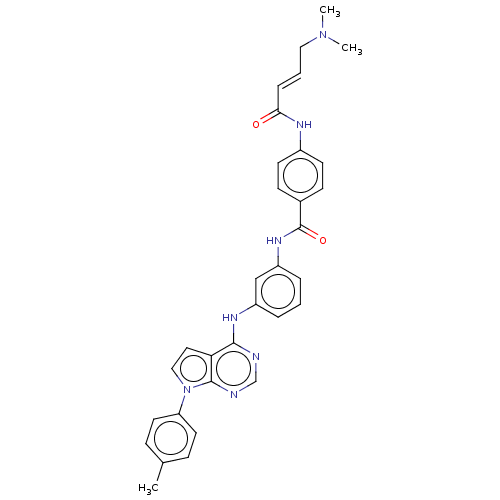
(CHEMBL4439287)Show SMILES CN(C)C\C=C\C(=O)Nc1ccc(cc1)C(=O)Nc1cccc(Nc2ncnc3n(ccc23)-c2ccc(C)cc2)c1 Show InChI InChI=1S/C32H31N7O2/c1-22-9-15-27(16-10-22)39-19-17-28-30(33-21-34-31(28)39)36-25-6-4-7-26(20-25)37-32(41)23-11-13-24(14-12-23)35-29(40)8-5-18-38(2)3/h4-17,19-21H,18H2,1-3H3,(H,35,40)(H,37,41)(H,33,34,36)/b8-5+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of PI5P4Kalpha (unknown origin) incubated for 15 mins in presence of ATP by ADP-Glo assay |
ACS Med Chem Lett 11: 346-352 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00402
BindingDB Entry DOI: 10.7270/Q2TM7FDP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha
(Homo sapiens) | BDBM50511410

(CHEMBL4284040)Show InChI InChI=1S/C20H13N3/c21-11-16(19-13-23-20-7-2-1-6-18(19)20)10-14-4-3-5-15-12-22-9-8-17(14)15/h1-10,12-13,23H/b16-10+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of PI5P4Kalpha (unknown origin) incubated for 15 mins in presence of ATP by ADP-Glo assay |
ACS Med Chem Lett 11: 346-352 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00402
BindingDB Entry DOI: 10.7270/Q2TM7FDP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha
(Homo sapiens) | BDBM50511425

(CHEMBL4572941)Show SMILES CN(C)C\C=C\C(=O)Nc1ccc(cc1)C(=O)Nc1ccc(Nc2cc(ncn2)-c2cnc3[nH]ccc3c2)cc1 Show InChI InChI=1S/C30H28N8O2/c1-38(2)15-3-4-28(39)36-24-7-5-20(6-8-24)30(40)37-25-11-9-23(10-12-25)35-27-17-26(33-19-34-27)22-16-21-13-14-31-29(21)32-18-22/h3-14,16-19H,15H2,1-2H3,(H,31,32)(H,36,39)(H,37,40)(H,33,34,35)/b4-3+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of PI5P4Kalpha (unknown origin) incubated for 15 mins in presence of ATP by ADP-Glo assay |
ACS Med Chem Lett 11: 346-352 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00402
BindingDB Entry DOI: 10.7270/Q2TM7FDP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha
(Homo sapiens) | BDBM50511419
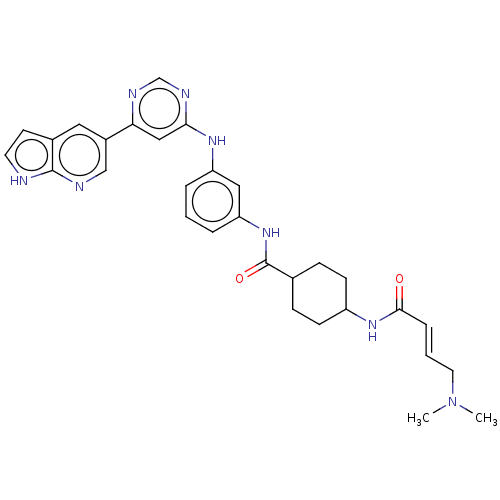
(CHEMBL4517979)Show SMILES CN(C)C\C=C\C(=O)NC1CCC(CC1)C(=O)Nc1cccc(Nc2cc(ncn2)-c2cnc3[nH]ccc3c2)c1 |(4.78,-11.85,;6.12,-11.08,;6.12,-9.54,;7.45,-11.85,;8.78,-11.08,;10.12,-11.85,;11.45,-11.08,;11.45,-9.54,;12.78,-11.85,;14.11,-11.08,;15.44,-11.85,;16.78,-11.08,;16.78,-9.54,;15.44,-8.77,;14.11,-9.54,;18.11,-8.77,;18.11,-7.23,;19.44,-9.54,;20.77,-8.77,;20.77,-7.23,;22.11,-6.46,;23.44,-7.24,;23.44,-8.76,;24.78,-9.53,;26.11,-8.76,;27.45,-9.54,;28.78,-8.76,;28.78,-7.23,;27.44,-6.46,;26.11,-7.22,;30.11,-9.53,;30.11,-11.08,;31.44,-11.84,;32.77,-11.07,;34.24,-11.54,;35.14,-10.3,;34.24,-9.05,;32.77,-9.53,;31.44,-8.76,;22.12,-9.54,)| Show InChI InChI=1S/C30H34N8O2/c1-38(2)14-4-7-28(39)36-23-10-8-20(9-11-23)30(40)37-25-6-3-5-24(16-25)35-27-17-26(33-19-34-27)22-15-21-12-13-31-29(21)32-18-22/h3-7,12-13,15-20,23H,8-11,14H2,1-2H3,(H,31,32)(H,36,39)(H,37,40)(H,33,34,35)/b7-4+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of PI5P4Kalpha (unknown origin) incubated for 15 mins in presence of ATP by ADP-Glo assay |
ACS Med Chem Lett 11: 346-352 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00402
BindingDB Entry DOI: 10.7270/Q2TM7FDP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha
(Homo sapiens) | BDBM50511418
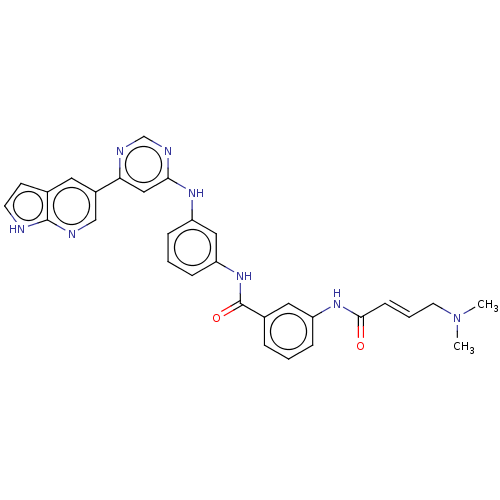
(CHEMBL4533123)Show SMILES CN(C)C\C=C\C(=O)Nc1cccc(c1)C(=O)Nc1cccc(Nc2cc(ncn2)-c2cnc3[nH]ccc3c2)c1 Show InChI InChI=1S/C30H28N8O2/c1-38(2)13-5-10-28(39)36-23-7-3-6-21(15-23)30(40)37-25-9-4-8-24(16-25)35-27-17-26(33-19-34-27)22-14-20-11-12-31-29(20)32-18-22/h3-12,14-19H,13H2,1-2H3,(H,31,32)(H,36,39)(H,37,40)(H,33,34,35)/b10-5+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of PI5P4Kalpha (unknown origin) incubated for 15 mins in presence of ATP by ADP-Glo assay |
ACS Med Chem Lett 11: 346-352 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00402
BindingDB Entry DOI: 10.7270/Q2TM7FDP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha
(Homo sapiens) | BDBM50511413
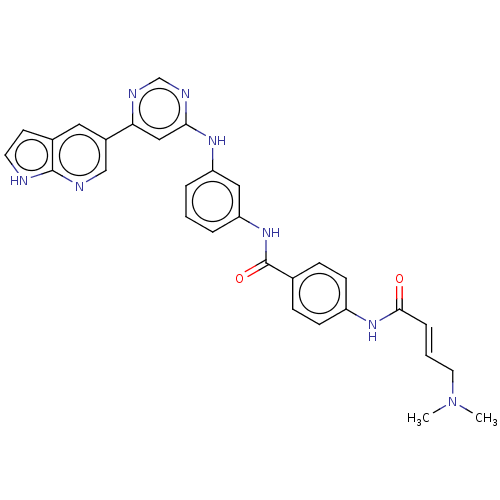
(CHEMBL4571099)Show SMILES CN(C)C\C=C\C(=O)Nc1ccc(cc1)C(=O)Nc1cccc(Nc2cc(ncn2)-c2cnc3[nH]ccc3c2)c1 Show InChI InChI=1S/C30H28N8O2/c1-38(2)14-4-7-28(39)36-23-10-8-20(9-11-23)30(40)37-25-6-3-5-24(16-25)35-27-17-26(33-19-34-27)22-15-21-12-13-31-29(21)32-18-22/h3-13,15-19H,14H2,1-2H3,(H,31,32)(H,36,39)(H,37,40)(H,33,34,35)/b7-4+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of PI5P4Kalpha (unknown origin) incubated for 15 mins in presence of ATP by ADP-Glo assay |
ACS Med Chem Lett 11: 346-352 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00402
BindingDB Entry DOI: 10.7270/Q2TM7FDP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha
(Homo sapiens) | BDBM50511423
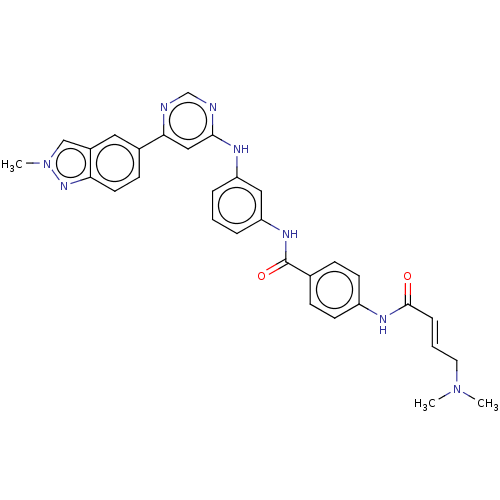
(CHEMBL4454740)Show SMILES CN(C)C\C=C\C(=O)Nc1ccc(cc1)C(=O)Nc1cccc(Nc2cc(ncn2)-c2ccc3nn(C)cc3c2)c1 Show InChI InChI=1S/C31H30N8O2/c1-38(2)15-5-8-30(40)35-24-12-9-21(10-13-24)31(41)36-26-7-4-6-25(17-26)34-29-18-28(32-20-33-29)22-11-14-27-23(16-22)19-39(3)37-27/h4-14,16-20H,15H2,1-3H3,(H,35,40)(H,36,41)(H,32,33,34)/b8-5+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of PI5P4Kalpha (unknown origin) incubated for 15 mins in presence of ATP by ADP-Glo assay |
ACS Med Chem Lett 11: 346-352 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00402
BindingDB Entry DOI: 10.7270/Q2TM7FDP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 beta
(Homo sapiens (Human)) | BDBM50511409
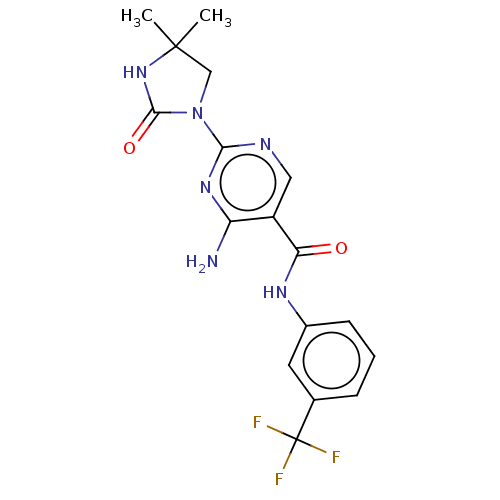
(Imanixil)Show SMILES CC1(C)CN(C(=O)N1)c1ncc(C(=O)Nc2cccc(c2)C(F)(F)F)c(N)n1 Show InChI InChI=1S/C17H17F3N6O2/c1-16(2)8-26(15(28)25-16)14-22-7-11(12(21)24-14)13(27)23-10-5-3-4-9(6-10)17(18,19)20/h3-7H,8H2,1-2H3,(H,23,27)(H,25,28)(H2,21,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of PI5P4Kbeta (unknown origin) incubated for 15 mins in presence of ATP by ADP-Glo assay |
ACS Med Chem Lett 11: 346-352 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00402
BindingDB Entry DOI: 10.7270/Q2TM7FDP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha
(Homo sapiens) | BDBM50511412
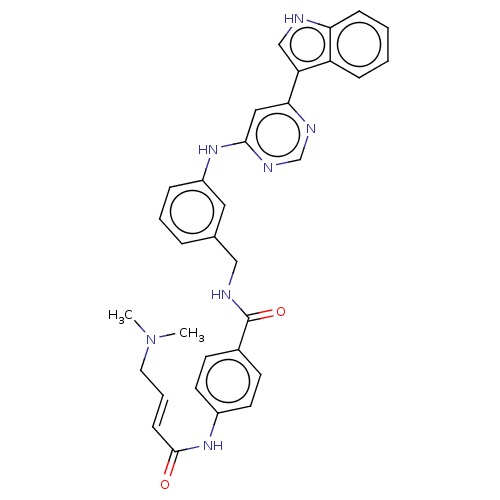
(CHEMBL4550598)Show SMILES CN(C)C\C=C\C(=O)Nc1ccc(cc1)C(=O)NCc1cccc(Nc2cc(ncn2)-c2c[nH]c3ccccc23)c1 Show InChI InChI=1S/C32H31N7O2/c1-39(2)16-6-11-31(40)38-24-14-12-23(13-15-24)32(41)34-19-22-7-5-8-25(17-22)37-30-18-29(35-21-36-30)27-20-33-28-10-4-3-9-26(27)28/h3-15,17-18,20-21,33H,16,19H2,1-2H3,(H,34,41)(H,38,40)(H,35,36,37)/b11-6+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of PI5P4Kalpha (unknown origin) incubated for 15 mins in presence of ATP by ADP-Glo assay |
ACS Med Chem Lett 11: 346-352 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00402
BindingDB Entry DOI: 10.7270/Q2TM7FDP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha
(Homo sapiens) | BDBM50511417
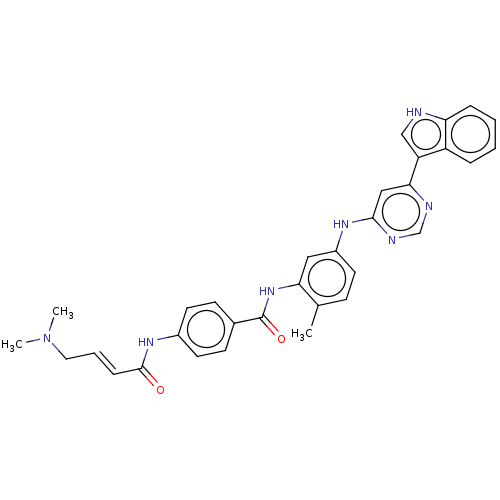
(CHEMBL4464636)Show SMILES CN(C)C\C=C\C(=O)Nc1ccc(cc1)C(=O)Nc1cc(Nc2cc(ncn2)-c2c[nH]c3ccccc23)ccc1C Show InChI InChI=1S/C32H31N7O2/c1-21-10-13-24(36-30-18-29(34-20-35-30)26-19-33-27-8-5-4-7-25(26)27)17-28(21)38-32(41)22-11-14-23(15-12-22)37-31(40)9-6-16-39(2)3/h4-15,17-20,33H,16H2,1-3H3,(H,37,40)(H,38,41)(H,34,35,36)/b9-6+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of PI5P4Kalpha (unknown origin) incubated for 15 mins in presence of ATP by ADP-Glo assay |
ACS Med Chem Lett 11: 346-352 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00402
BindingDB Entry DOI: 10.7270/Q2TM7FDP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 beta
(Homo sapiens (Human)) | BDBM50511422

(CHEMBL4446338)Show SMILES CN(C)C\C=C\C(=O)Nc1ccc(cc1)C(=O)Nc1cccc(Nc2cc(ncn2)-c2c[nH]c3ccccc23)c1 Show InChI InChI=1S/C31H29N7O2/c1-38(2)16-6-11-30(39)36-22-14-12-21(13-15-22)31(40)37-24-8-5-7-23(17-24)35-29-18-28(33-20-34-29)26-19-32-27-10-4-3-9-25(26)27/h3-15,17-20,32H,16H2,1-2H3,(H,36,39)(H,37,40)(H,33,34,35)/b11-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of PI5P4Kbeta (unknown origin) incubated for 15 mins in presence of ATP by ADP-Glo assay |
ACS Med Chem Lett 11: 346-352 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00402
BindingDB Entry DOI: 10.7270/Q2TM7FDP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 beta
(Homo sapiens (Human)) | BDBM50511415

(CHEMBL4461694)Show SMILES CN(C)C\C=C\C(=O)Nc1ccc(cc1)C(=O)Nc1cccc(Nc2cc(Nc3ccc(C)cc3)ncn2)c1 Show InChI InChI=1S/C30H31N7O2/c1-21-9-13-23(14-10-21)33-27-19-28(32-20-31-27)34-25-6-4-7-26(18-25)36-30(39)22-11-15-24(16-12-22)35-29(38)8-5-17-37(2)3/h4-16,18-20H,17H2,1-3H3,(H,35,38)(H,36,39)(H2,31,32,33,34)/b8-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of PI5P4Kbeta (unknown origin) incubated for 15 mins in presence of ATP by ADP-Glo assay |
ACS Med Chem Lett 11: 346-352 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00402
BindingDB Entry DOI: 10.7270/Q2TM7FDP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 beta
(Homo sapiens (Human)) | BDBM50511414

(CHEMBL4472811)Show SMILES CN(C)C\C=C\C(=O)Nc1ccc(cc1)C(=O)Nc1cccc(Nc2cc(Nc3ccc(cc3)-c3ccccc3)ncn2)c1 Show InChI InChI=1S/C35H33N7O2/c1-42(2)21-7-12-34(43)40-29-19-15-27(16-20-29)35(44)41-31-11-6-10-30(22-31)39-33-23-32(36-24-37-33)38-28-17-13-26(14-18-28)25-8-4-3-5-9-25/h3-20,22-24H,21H2,1-2H3,(H,40,43)(H,41,44)(H2,36,37,38,39)/b12-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of PI5P4Kbeta (unknown origin) incubated for 15 mins in presence of ATP by ADP-Glo assay |
ACS Med Chem Lett 11: 346-352 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00402
BindingDB Entry DOI: 10.7270/Q2TM7FDP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha
(Homo sapiens) | BDBM50511424
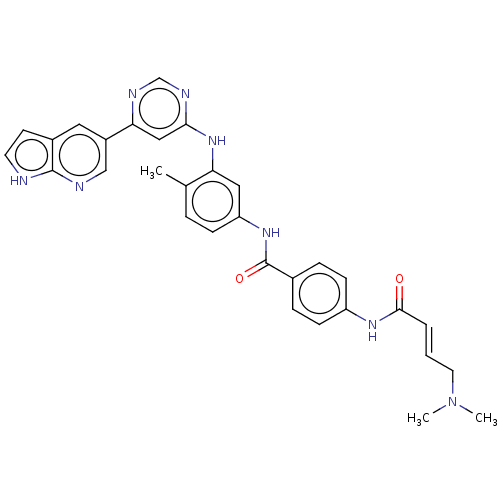
(CHEMBL4518868)Show SMILES CN(C)C\C=C\C(=O)Nc1ccc(cc1)C(=O)Nc1ccc(C)c(Nc2cc(ncn2)-c2cnc3[nH]ccc3c2)c1 Show InChI InChI=1S/C31H30N8O2/c1-20-6-9-25(37-31(41)21-7-10-24(11-8-21)36-29(40)5-4-14-39(2)3)16-26(20)38-28-17-27(34-19-35-28)23-15-22-12-13-32-30(22)33-18-23/h4-13,15-19H,14H2,1-3H3,(H,32,33)(H,36,40)(H,37,41)(H,34,35,38)/b5-4+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of PI5P4Kalpha (unknown origin) incubated for 15 mins in presence of ATP by ADP-Glo assay |
ACS Med Chem Lett 11: 346-352 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00402
BindingDB Entry DOI: 10.7270/Q2TM7FDP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 beta
(Homo sapiens (Human)) | BDBM50511418

(CHEMBL4533123)Show SMILES CN(C)C\C=C\C(=O)Nc1cccc(c1)C(=O)Nc1cccc(Nc2cc(ncn2)-c2cnc3[nH]ccc3c2)c1 Show InChI InChI=1S/C30H28N8O2/c1-38(2)13-5-10-28(39)36-23-7-3-6-21(15-23)30(40)37-25-9-4-8-24(16-25)35-27-17-26(33-19-34-27)22-14-20-11-12-31-29(20)32-18-22/h3-12,14-19H,13H2,1-2H3,(H,31,32)(H,36,39)(H,37,40)(H,33,34,35)/b10-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of PI5P4Kbeta (unknown origin) incubated for 15 mins in presence of ATP by ADP-Glo assay |
ACS Med Chem Lett 11: 346-352 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00402
BindingDB Entry DOI: 10.7270/Q2TM7FDP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 beta
(Homo sapiens (Human)) | BDBM50511421

(CHEMBL4439287)Show SMILES CN(C)C\C=C\C(=O)Nc1ccc(cc1)C(=O)Nc1cccc(Nc2ncnc3n(ccc23)-c2ccc(C)cc2)c1 Show InChI InChI=1S/C32H31N7O2/c1-22-9-15-27(16-10-22)39-19-17-28-30(33-21-34-31(28)39)36-25-6-4-7-26(20-25)37-32(41)23-11-13-24(14-12-23)35-29(40)8-5-18-38(2)3/h4-17,19-21H,18H2,1-3H3,(H,35,40)(H,37,41)(H,33,34,36)/b8-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of PI5P4Kbeta (unknown origin) incubated for 15 mins in presence of ATP by ADP-Glo assay |
ACS Med Chem Lett 11: 346-352 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00402
BindingDB Entry DOI: 10.7270/Q2TM7FDP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 beta
(Homo sapiens (Human)) | BDBM50511420

(CHEMBL4449493)Show SMILES CN(C)C\C=C\C(=O)NC1CCN(CC1)C(=O)Cc1cccc(Nc2cc(ncn2)-c2cnc3[nH]ccc3c2)c1 Show InChI InChI=1S/C30H34N8O2/c1-37(2)12-4-7-28(39)36-24-9-13-38(14-10-24)29(40)16-21-5-3-6-25(15-21)35-27-18-26(33-20-34-27)23-17-22-8-11-31-30(22)32-19-23/h3-8,11,15,17-20,24H,9-10,12-14,16H2,1-2H3,(H,31,32)(H,36,39)(H,33,34,35)/b7-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of PI5P4Kbeta (unknown origin) incubated for 15 mins in presence of ATP by ADP-Glo assay |
ACS Med Chem Lett 11: 346-352 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00402
BindingDB Entry DOI: 10.7270/Q2TM7FDP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 beta
(Homo sapiens (Human)) | BDBM50511413

(CHEMBL4571099)Show SMILES CN(C)C\C=C\C(=O)Nc1ccc(cc1)C(=O)Nc1cccc(Nc2cc(ncn2)-c2cnc3[nH]ccc3c2)c1 Show InChI InChI=1S/C30H28N8O2/c1-38(2)14-4-7-28(39)36-23-10-8-20(9-11-23)30(40)37-25-6-3-5-24(16-25)35-27-17-26(33-19-34-27)22-15-21-12-13-31-29(21)32-18-22/h3-13,15-19H,14H2,1-2H3,(H,31,32)(H,36,39)(H,37,40)(H,33,34,35)/b7-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of PI5P4Kbeta (unknown origin) incubated for 15 mins in presence of ATP by ADP-Glo assay |
ACS Med Chem Lett 11: 346-352 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00402
BindingDB Entry DOI: 10.7270/Q2TM7FDP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 beta
(Homo sapiens (Human)) | BDBM50511423

(CHEMBL4454740)Show SMILES CN(C)C\C=C\C(=O)Nc1ccc(cc1)C(=O)Nc1cccc(Nc2cc(ncn2)-c2ccc3nn(C)cc3c2)c1 Show InChI InChI=1S/C31H30N8O2/c1-38(2)15-5-8-30(40)35-24-12-9-21(10-13-24)31(41)36-26-7-4-6-25(17-26)34-29-18-28(32-20-33-29)22-11-14-27-23(16-22)19-39(3)37-27/h4-14,16-20H,15H2,1-3H3,(H,35,40)(H,36,41)(H,32,33,34)/b8-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of PI5P4Kbeta (unknown origin) incubated for 15 mins in presence of ATP by ADP-Glo assay |
ACS Med Chem Lett 11: 346-352 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00402
BindingDB Entry DOI: 10.7270/Q2TM7FDP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 gamma
(Homo sapiens (Human)) | BDBM50511426
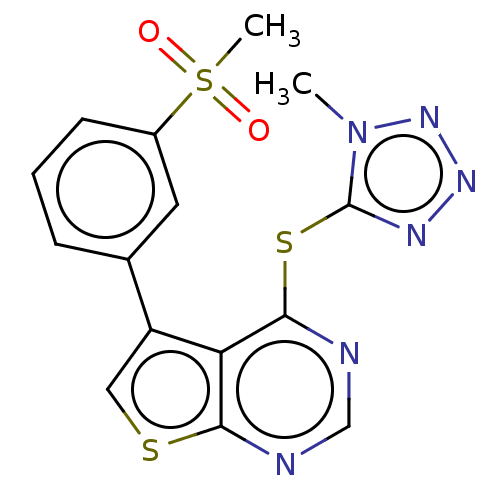
(CHEMBL1378392)Show SMILES Cn1nnnc1Sc1ncnc2scc(-c3cccc(c3)S(C)(=O)=O)c12 Show InChI InChI=1S/C15H12N6O2S3/c1-21-15(18-19-20-21)25-14-12-11(7-24-13(12)16-8-17-14)9-4-3-5-10(6-9)26(2,22)23/h3-8H,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of PI5P4Kgamma (unknown origin) |
ACS Med Chem Lett 11: 346-352 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00402
BindingDB Entry DOI: 10.7270/Q2TM7FDP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha
(Homo sapiens) | BDBM50511411
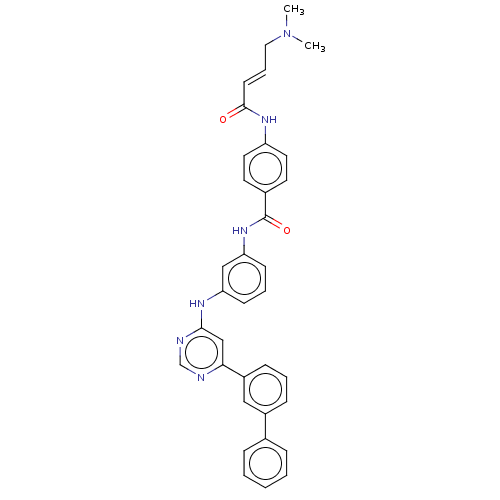
(CHEMBL4563077)Show SMILES CN(C)C\C=C\C(=O)Nc1ccc(cc1)C(=O)Nc1cccc(Nc2cc(ncn2)-c2cccc(c2)-c2ccccc2)c1 Show InChI InChI=1S/C35H32N6O2/c1-41(2)20-8-15-34(42)39-29-18-16-26(17-19-29)35(43)40-31-14-7-13-30(22-31)38-33-23-32(36-24-37-33)28-12-6-11-27(21-28)25-9-4-3-5-10-25/h3-19,21-24H,20H2,1-2H3,(H,39,42)(H,40,43)(H,36,37,38)/b15-8+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of PI5P4Kalpha (unknown origin) incubated for 15 mins in presence of ATP by ADP-Glo assay |
ACS Med Chem Lett 11: 346-352 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00402
BindingDB Entry DOI: 10.7270/Q2TM7FDP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha
(Homo sapiens) | BDBM50511416
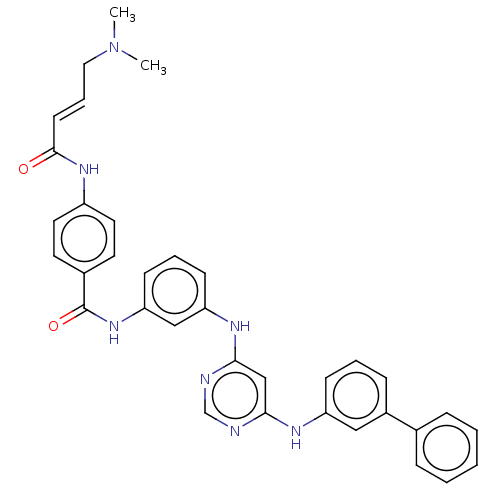
(CHEMBL4537067)Show SMILES CN(C)C\C=C\C(=O)Nc1ccc(cc1)C(=O)Nc1cccc(Nc2cc(Nc3cccc(c3)-c3ccccc3)ncn2)c1 Show InChI InChI=1S/C35H33N7O2/c1-42(2)20-8-15-34(43)40-28-18-16-26(17-19-28)35(44)41-31-14-7-13-30(22-31)39-33-23-32(36-24-37-33)38-29-12-6-11-27(21-29)25-9-4-3-5-10-25/h3-19,21-24H,20H2,1-2H3,(H,40,43)(H,41,44)(H2,36,37,38,39)/b15-8+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of PI5P4Kalpha (unknown origin) incubated for 15 mins in presence of ATP by ADP-Glo assay |
ACS Med Chem Lett 11: 346-352 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00402
BindingDB Entry DOI: 10.7270/Q2TM7FDP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 beta
(Homo sapiens (Human)) | BDBM50511424

(CHEMBL4518868)Show SMILES CN(C)C\C=C\C(=O)Nc1ccc(cc1)C(=O)Nc1ccc(C)c(Nc2cc(ncn2)-c2cnc3[nH]ccc3c2)c1 Show InChI InChI=1S/C31H30N8O2/c1-20-6-9-25(37-31(41)21-7-10-24(11-8-21)36-29(40)5-4-14-39(2)3)16-26(20)38-28-17-27(34-19-35-28)23-15-22-12-13-32-30(22)33-18-23/h4-13,15-19H,14H2,1-3H3,(H,32,33)(H,36,40)(H,37,41)(H,34,35,38)/b5-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of PI5P4Kbeta (unknown origin) incubated for 15 mins in presence of ATP by ADP-Glo assay |
ACS Med Chem Lett 11: 346-352 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00402
BindingDB Entry DOI: 10.7270/Q2TM7FDP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 beta
(Homo sapiens (Human)) | BDBM50511416

(CHEMBL4537067)Show SMILES CN(C)C\C=C\C(=O)Nc1ccc(cc1)C(=O)Nc1cccc(Nc2cc(Nc3cccc(c3)-c3ccccc3)ncn2)c1 Show InChI InChI=1S/C35H33N7O2/c1-42(2)20-8-15-34(43)40-28-18-16-26(17-19-28)35(44)41-31-14-7-13-30(22-31)39-33-23-32(36-24-37-33)38-29-12-6-11-27(21-29)25-9-4-3-5-10-25/h3-19,21-24H,20H2,1-2H3,(H,40,43)(H,41,44)(H2,36,37,38,39)/b15-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of PI5P4Kbeta (unknown origin) incubated for 15 mins in presence of ATP by ADP-Glo assay |
ACS Med Chem Lett 11: 346-352 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00402
BindingDB Entry DOI: 10.7270/Q2TM7FDP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 beta
(Homo sapiens (Human)) | BDBM50511412

(CHEMBL4550598)Show SMILES CN(C)C\C=C\C(=O)Nc1ccc(cc1)C(=O)NCc1cccc(Nc2cc(ncn2)-c2c[nH]c3ccccc23)c1 Show InChI InChI=1S/C32H31N7O2/c1-39(2)16-6-11-31(40)38-24-14-12-23(13-15-24)32(41)34-19-22-7-5-8-25(17-22)37-30-18-29(35-21-36-30)27-20-33-28-10-4-3-9-26(27)28/h3-15,17-18,20-21,33H,16,19H2,1-2H3,(H,34,41)(H,38,40)(H,35,36,37)/b11-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of PI5P4Kbeta (unknown origin) incubated for 15 mins in presence of ATP by ADP-Glo assay |
ACS Med Chem Lett 11: 346-352 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00402
BindingDB Entry DOI: 10.7270/Q2TM7FDP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 beta
(Homo sapiens (Human)) | BDBM50511411

(CHEMBL4563077)Show SMILES CN(C)C\C=C\C(=O)Nc1ccc(cc1)C(=O)Nc1cccc(Nc2cc(ncn2)-c2cccc(c2)-c2ccccc2)c1 Show InChI InChI=1S/C35H32N6O2/c1-41(2)20-8-15-34(42)39-29-18-16-26(17-19-29)35(43)40-31-14-7-13-30(22-31)38-33-23-32(36-24-37-33)28-12-6-11-27(21-28)25-9-4-3-5-10-25/h3-19,21-24H,20H2,1-2H3,(H,39,42)(H,40,43)(H,36,37,38)/b15-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of PI5P4Kbeta (unknown origin) incubated for 15 mins in presence of ATP by ADP-Glo assay |
ACS Med Chem Lett 11: 346-352 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00402
BindingDB Entry DOI: 10.7270/Q2TM7FDP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 beta
(Homo sapiens (Human)) | BDBM50511419

(CHEMBL4517979)Show SMILES CN(C)C\C=C\C(=O)NC1CCC(CC1)C(=O)Nc1cccc(Nc2cc(ncn2)-c2cnc3[nH]ccc3c2)c1 |(4.78,-11.85,;6.12,-11.08,;6.12,-9.54,;7.45,-11.85,;8.78,-11.08,;10.12,-11.85,;11.45,-11.08,;11.45,-9.54,;12.78,-11.85,;14.11,-11.08,;15.44,-11.85,;16.78,-11.08,;16.78,-9.54,;15.44,-8.77,;14.11,-9.54,;18.11,-8.77,;18.11,-7.23,;19.44,-9.54,;20.77,-8.77,;20.77,-7.23,;22.11,-6.46,;23.44,-7.24,;23.44,-8.76,;24.78,-9.53,;26.11,-8.76,;27.45,-9.54,;28.78,-8.76,;28.78,-7.23,;27.44,-6.46,;26.11,-7.22,;30.11,-9.53,;30.11,-11.08,;31.44,-11.84,;32.77,-11.07,;34.24,-11.54,;35.14,-10.3,;34.24,-9.05,;32.77,-9.53,;31.44,-8.76,;22.12,-9.54,)| Show InChI InChI=1S/C30H34N8O2/c1-38(2)14-4-7-28(39)36-23-10-8-20(9-11-23)30(40)37-25-6-3-5-24(16-25)35-27-17-26(33-19-34-27)22-15-21-12-13-31-29(21)32-18-22/h3-7,12-13,15-20,23H,8-11,14H2,1-2H3,(H,31,32)(H,36,39)(H,37,40)(H,33,34,35)/b7-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of PI5P4Kbeta (unknown origin) incubated for 15 mins in presence of ATP by ADP-Glo assay |
ACS Med Chem Lett 11: 346-352 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00402
BindingDB Entry DOI: 10.7270/Q2TM7FDP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 beta
(Homo sapiens (Human)) | BDBM50511417

(CHEMBL4464636)Show SMILES CN(C)C\C=C\C(=O)Nc1ccc(cc1)C(=O)Nc1cc(Nc2cc(ncn2)-c2c[nH]c3ccccc23)ccc1C Show InChI InChI=1S/C32H31N7O2/c1-21-10-13-24(36-30-18-29(34-20-35-30)26-19-33-27-8-5-4-7-25(26)27)17-28(21)38-32(41)22-11-14-23(15-12-22)37-31(40)9-6-16-39(2)3/h4-15,17-20,33H,16H2,1-3H3,(H,37,40)(H,38,41)(H,34,35,36)/b9-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of PI5P4Kbeta (unknown origin) incubated for 15 mins in presence of ATP by ADP-Glo assay |
ACS Med Chem Lett 11: 346-352 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00402
BindingDB Entry DOI: 10.7270/Q2TM7FDP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 beta
(Homo sapiens (Human)) | BDBM50511425

(CHEMBL4572941)Show SMILES CN(C)C\C=C\C(=O)Nc1ccc(cc1)C(=O)Nc1ccc(Nc2cc(ncn2)-c2cnc3[nH]ccc3c2)cc1 Show InChI InChI=1S/C30H28N8O2/c1-38(2)15-3-4-28(39)36-24-7-5-20(6-8-24)30(40)37-25-11-9-23(10-12-25)35-27-17-26(33-19-34-27)22-16-21-13-14-31-29(21)32-18-22/h3-14,16-19H,15H2,1-2H3,(H,31,32)(H,36,39)(H,37,40)(H,33,34,35)/b4-3+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of PI5P4Kbeta (unknown origin) incubated for 15 mins in presence of ATP by ADP-Glo assay |
ACS Med Chem Lett 11: 346-352 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00402
BindingDB Entry DOI: 10.7270/Q2TM7FDP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data