

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50491052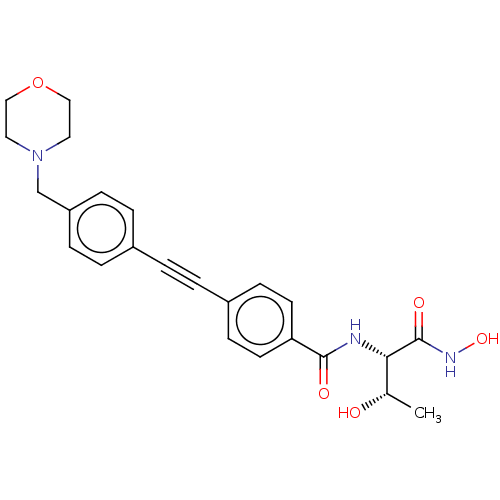 (CHEMBL2377693) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli str. K-12 substr. MG1655 LpxC using UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 20... | Bioorg Med Chem Lett 23: 2362-7 (2013) Article DOI: 10.1016/j.bmcl.2013.02.055 BindingDB Entry DOI: 10.7270/Q2F76GGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50491048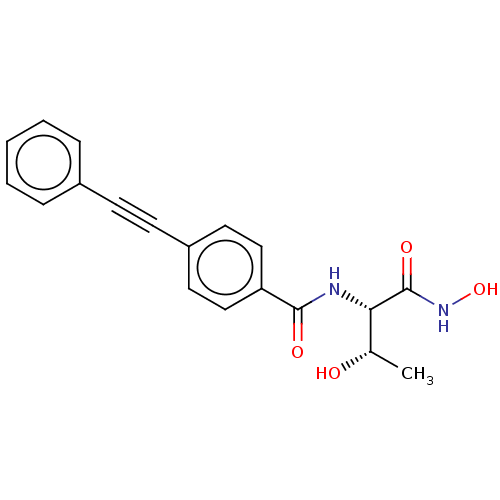 (CHEMBL2377694) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli str. K-12 substr. MG1655 LpxC using UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 20... | Bioorg Med Chem Lett 23: 2362-7 (2013) Article DOI: 10.1016/j.bmcl.2013.02.055 BindingDB Entry DOI: 10.7270/Q2F76GGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50491047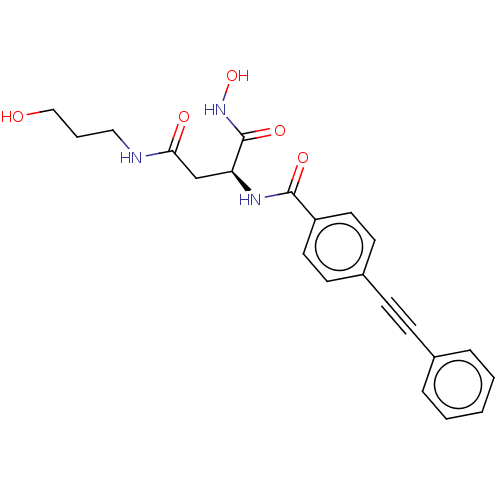 (CHEMBL2377569) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli str. K-12 substr. MG1655 LpxC using UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 20... | Bioorg Med Chem Lett 23: 2362-7 (2013) Article DOI: 10.1016/j.bmcl.2013.02.055 BindingDB Entry DOI: 10.7270/Q2F76GGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50491046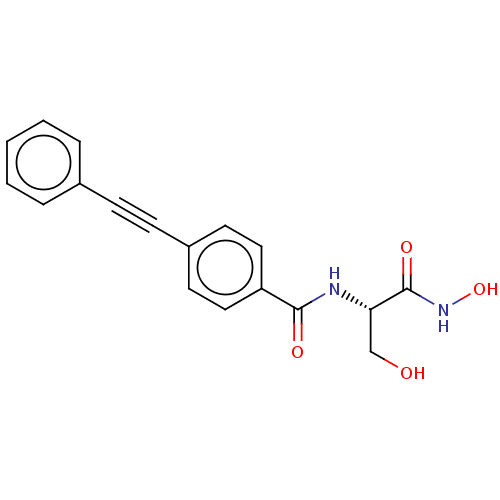 (CHEMBL2377699) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli str. K-12 substr. MG1655 LpxC using UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 20... | Bioorg Med Chem Lett 23: 2362-7 (2013) Article DOI: 10.1016/j.bmcl.2013.02.055 BindingDB Entry DOI: 10.7270/Q2F76GGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50491056 (CHEMBL2377567) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli str. K-12 substr. MG1655 LpxC using UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 20... | Bioorg Med Chem Lett 23: 2362-7 (2013) Article DOI: 10.1016/j.bmcl.2013.02.055 BindingDB Entry DOI: 10.7270/Q2F76GGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50491045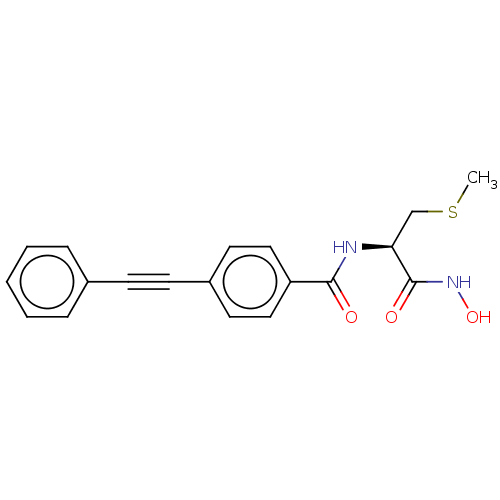 (CHEMBL2377702) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli str. K-12 substr. MG1655 LpxC using UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 20... | Bioorg Med Chem Lett 23: 2362-7 (2013) Article DOI: 10.1016/j.bmcl.2013.02.055 BindingDB Entry DOI: 10.7270/Q2F76GGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50491051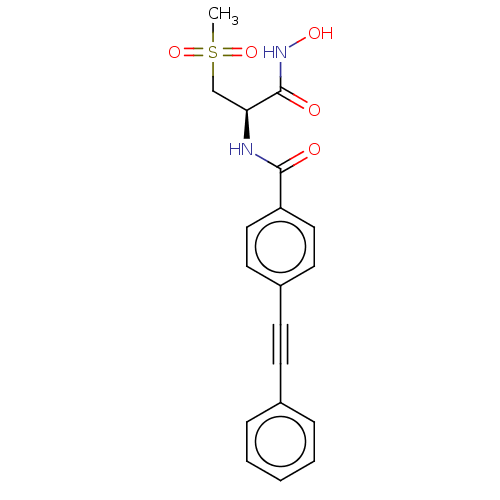 (CHEMBL2377703) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli str. K-12 substr. MG1655 LpxC using UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 20... | Bioorg Med Chem Lett 23: 2362-7 (2013) Article DOI: 10.1016/j.bmcl.2013.02.055 BindingDB Entry DOI: 10.7270/Q2F76GGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50491044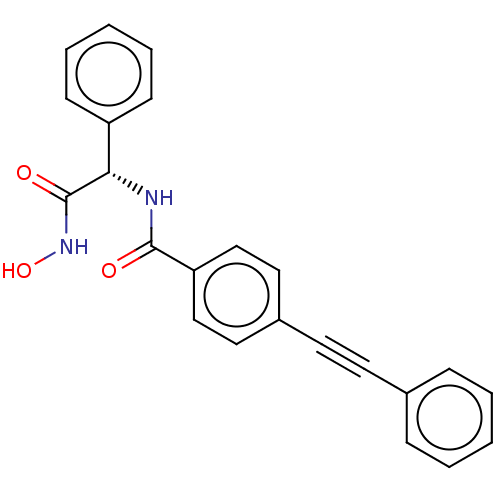 (CHEMBL2377704) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli str. K-12 substr. MG1655 LpxC using UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 20... | Bioorg Med Chem Lett 23: 2362-7 (2013) Article DOI: 10.1016/j.bmcl.2013.02.055 BindingDB Entry DOI: 10.7270/Q2F76GGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50210717 (CHEMBL397275 | N1-((2S,3R)-4-(3-ethylbenzylamino)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 17: 3378-83 (2007) Article DOI: 10.1016/j.bmcl.2007.03.096 BindingDB Entry DOI: 10.7270/Q2RB748S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phenylalanine--tRNA ligase alpha/beta subunit (Escherichia coli (Enterobacteria)) | BDBM231666 (N-(2-(1H-indol-3-yl)ethyl)-3-(3-thiazol-2-ylureido...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | 8.0 | n/a |
AstraZeneca R&D Boston | Assay Description Compounds were solubilized in DMSO. Serial 2-fold dilutions covering two concentration ranges, 10 mM to 19.5 μM and 100 μM to 195 nM, were ... | J Biol Chem 289: 21651-62 (2014) Article DOI: 10.1074/jbc.M114.574061 BindingDB Entry DOI: 10.7270/Q2BG2MVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phenylalanine--tRNA ligase alpha/beta subunit (Escherichia coli (Enterobacteria)) | BDBM231665 (1-(3-(4-(Pyridin-2-yl)piperazin-1-ylsulfonyl)pheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | 8.0 | n/a |
AstraZeneca R&D Boston | Assay Description Compounds were solubilized in DMSO. Serial 2-fold dilutions covering two concentration ranges, 10 mM to 19.5 μM and 100 μM to 195 nM, were ... | J Biol Chem 289: 21651-62 (2014) Article DOI: 10.1074/jbc.M114.574061 BindingDB Entry DOI: 10.7270/Q2BG2MVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50491050 (CHEMBL2377698) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli str. K-12 substr. MG1655 LpxC using UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 20... | Bioorg Med Chem Lett 23: 2362-7 (2013) Article DOI: 10.1016/j.bmcl.2013.02.055 BindingDB Entry DOI: 10.7270/Q2F76GGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50491055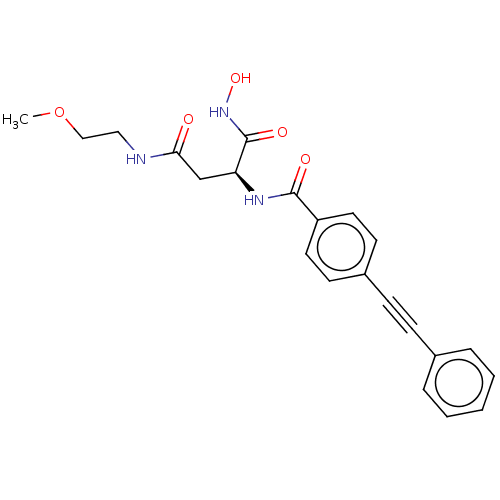 (CHEMBL2377570) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli str. K-12 substr. MG1655 LpxC using UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 20... | Bioorg Med Chem Lett 23: 2362-7 (2013) Article DOI: 10.1016/j.bmcl.2013.02.055 BindingDB Entry DOI: 10.7270/Q2F76GGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50491043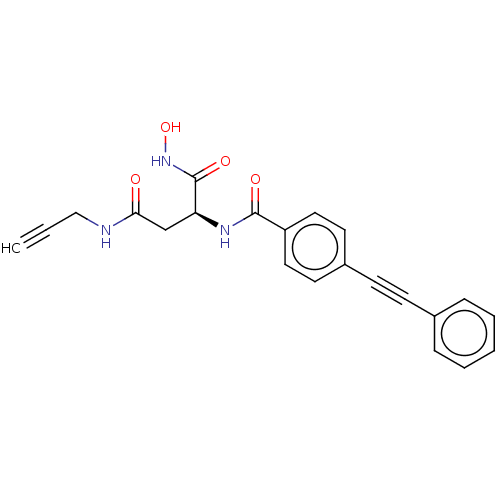 (CHEMBL2377571) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli str. K-12 substr. MG1655 LpxC using UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 20... | Bioorg Med Chem Lett 23: 2362-7 (2013) Article DOI: 10.1016/j.bmcl.2013.02.055 BindingDB Entry DOI: 10.7270/Q2F76GGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50491058 (CHEMBL2377690) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli str. K-12 substr. MG1655 LpxC using UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 20... | Bioorg Med Chem Lett 23: 2362-7 (2013) Article DOI: 10.1016/j.bmcl.2013.02.055 BindingDB Entry DOI: 10.7270/Q2F76GGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50210721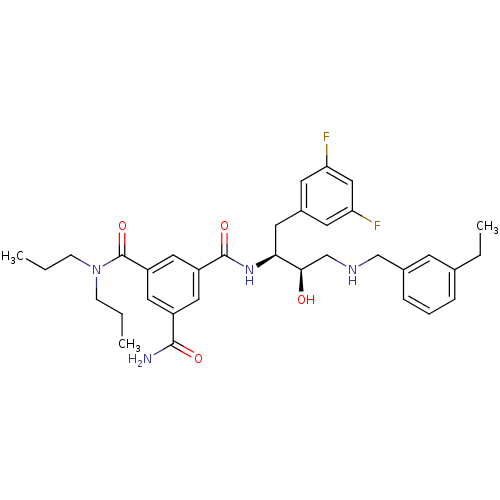 (CHEMBL397714 | N1-((2S,3R)-4-(3-ethylbenzylamino)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 17: 3378-83 (2007) Article DOI: 10.1016/j.bmcl.2007.03.096 BindingDB Entry DOI: 10.7270/Q2RB748S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM15797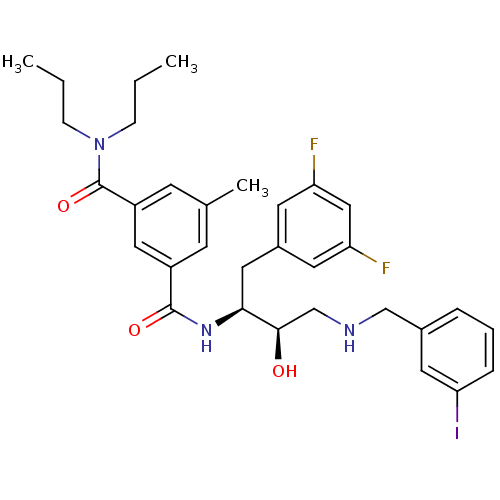 ((1S,2R)-N-[1-(3,5-Difluorobenzyl)-2-hydroxy-3-(3-i...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.8 | 37 |
Elan Pharmaceuticals | Assay Description Beta-cleavage ELISA assays were carried out in reaction buffer containing enzyme, substrate MBP-C125, and test compounds. Generated beta-cleaved prod... | J Med Chem 50: 776-81 (2007) Article DOI: 10.1021/jm061242y BindingDB Entry DOI: 10.7270/Q2K935TZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50491053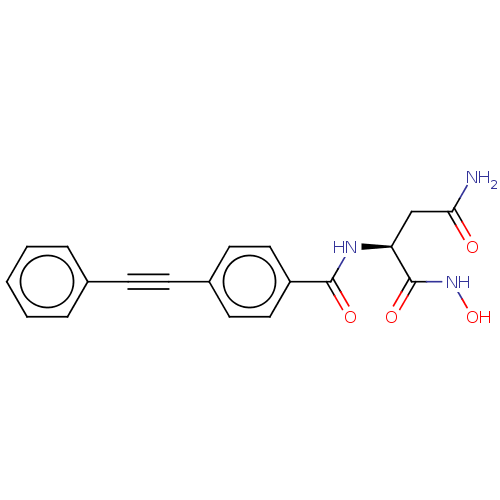 (CHEMBL2377568) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli str. K-12 substr. MG1655 LpxC using UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 20... | Bioorg Med Chem Lett 23: 2362-7 (2013) Article DOI: 10.1016/j.bmcl.2013.02.055 BindingDB Entry DOI: 10.7270/Q2F76GGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50210709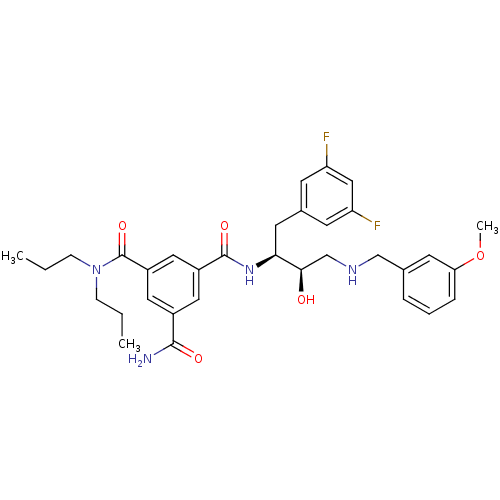 (CHEMBL396765 | N1-((2S,3R)-4-(3-methoxybenzylamino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 17: 3378-83 (2007) Article DOI: 10.1016/j.bmcl.2007.03.096 BindingDB Entry DOI: 10.7270/Q2RB748S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50491042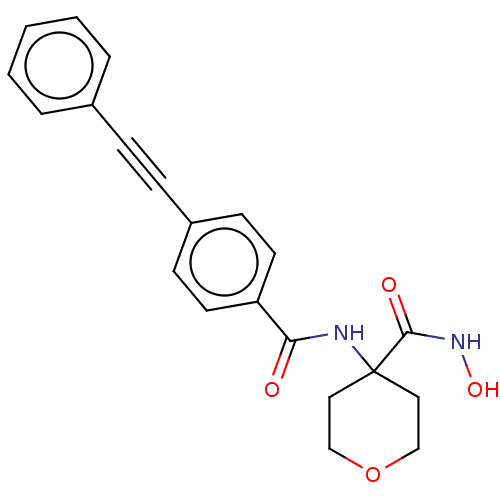 (CHEMBL2377688) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli str. K-12 substr. MG1655 LpxC using UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 20... | Bioorg Med Chem Lett 23: 2362-7 (2013) Article DOI: 10.1016/j.bmcl.2013.02.055 BindingDB Entry DOI: 10.7270/Q2F76GGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phenylalanine--tRNA ligase alpha/beta subunit (Pseudomonas aeruginosa) | BDBM231666 (N-(2-(1H-indol-3-yl)ethyl)-3-(3-thiazol-2-ylureido...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 8.0 | n/a |
AstraZeneca R&D Boston | Assay Description Compounds were solubilized in DMSO. Serial 2-fold dilutions covering two concentration ranges, 10 mM to 19.5 μM and 100 μM to 195 nM, were ... | J Biol Chem 289: 21651-62 (2014) Article DOI: 10.1074/jbc.M114.574061 BindingDB Entry DOI: 10.7270/Q2BG2MVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50491059 (CHEMBL2377566) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli str. K-12 substr. MG1655 LpxC using UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 20... | Bioorg Med Chem Lett 23: 2362-7 (2013) Article DOI: 10.1016/j.bmcl.2013.02.055 BindingDB Entry DOI: 10.7270/Q2F76GGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM92261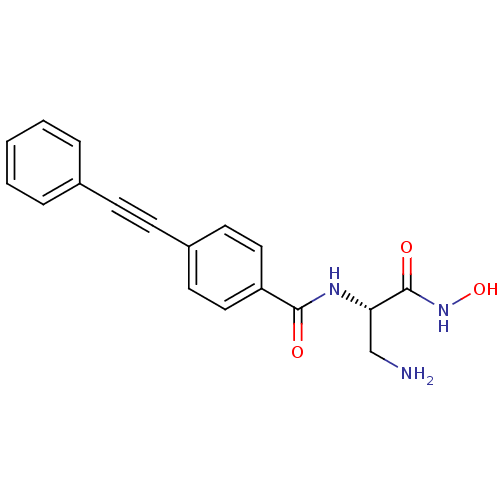 (CS251) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli str. K-12 substr. MG1655 LpxC using UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 20... | Bioorg Med Chem Lett 23: 2362-7 (2013) Article DOI: 10.1016/j.bmcl.2013.02.055 BindingDB Entry DOI: 10.7270/Q2F76GGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50491041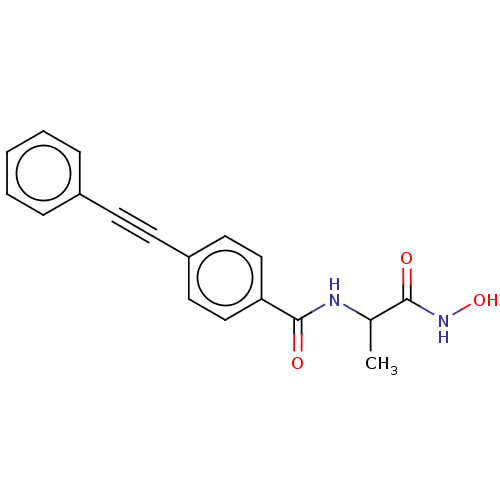 (CHEMBL2377696) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli str. K-12 substr. MG1655 LpxC using UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 20... | Bioorg Med Chem Lett 23: 2362-7 (2013) Article DOI: 10.1016/j.bmcl.2013.02.055 BindingDB Entry DOI: 10.7270/Q2F76GGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM15798 ((1S,2R)-N-[1-(3,5-Difluorobenzyl)-2-hydroxy-3-(3-m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 4.8 | 37 |
Elan Pharmaceuticals | Assay Description Beta-cleavage ELISA assays were carried out in reaction buffer containing enzyme, substrate MBP-C125, and test compounds. Generated beta-cleaved prod... | J Med Chem 50: 776-81 (2007) Article DOI: 10.1021/jm061242y BindingDB Entry DOI: 10.7270/Q2K935TZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Phenylalanine--tRNA ligase alpha/beta subunit (Pseudomonas aeruginosa) | BDBM231665 (1-(3-(4-(Pyridin-2-yl)piperazin-1-ylsulfonyl)pheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | 8.0 | n/a |
AstraZeneca R&D Boston | Assay Description Compounds were solubilized in DMSO. Serial 2-fold dilutions covering two concentration ranges, 10 mM to 19.5 μM and 100 μM to 195 nM, were ... | J Biol Chem 289: 21651-62 (2014) Article DOI: 10.1074/jbc.M114.574061 BindingDB Entry DOI: 10.7270/Q2BG2MVZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50491040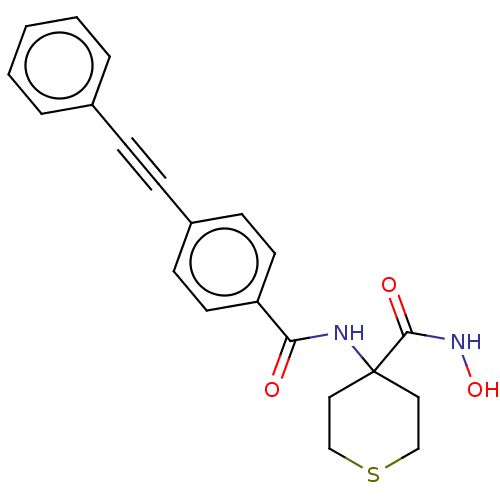 (CHEMBL2377689) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli str. K-12 substr. MG1655 LpxC using UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 20... | Bioorg Med Chem Lett 23: 2362-7 (2013) Article DOI: 10.1016/j.bmcl.2013.02.055 BindingDB Entry DOI: 10.7270/Q2F76GGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM15790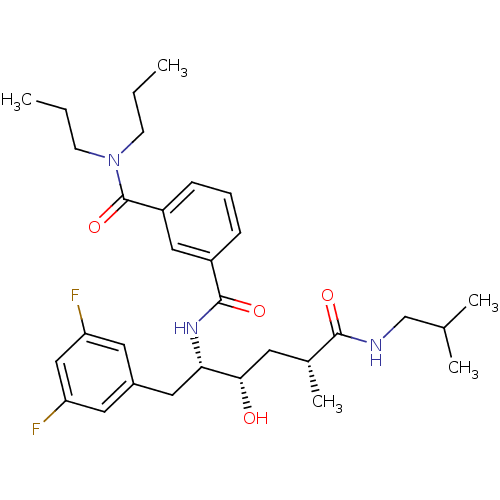 (3-N-[(2S,3S,5R)-1-(3,5-difluorophenyl)-3-hydroxy-5...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 4.8 | 37 |
Elan Pharmaceuticals | Assay Description Beta-cleavage ELISA assays were carried out in reaction buffer containing enzyme, substrate MBP-C125, and test compounds. Generated beta-cleaved prod... | J Med Chem 50: 776-81 (2007) Article DOI: 10.1021/jm061242y BindingDB Entry DOI: 10.7270/Q2K935TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50210719 (3-(((2S,3R)-4-(3-methoxybenzylamino)-3-hydroxy-1-p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 17: 3378-83 (2007) Article DOI: 10.1016/j.bmcl.2007.03.096 BindingDB Entry DOI: 10.7270/Q2RB748S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phenylalanine--tRNA ligase alpha/beta subunit (Escherichia coli (Enterobacteria)) | BDBM231670 (5-(3-Methoxyphenyl)-3-(trifluoromethyl)-1H-pyrazol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | 8.0 | n/a |
AstraZeneca R&D Boston | Assay Description Compounds were solubilized in DMSO. Serial 2-fold dilutions covering two concentration ranges, 10 mM to 19.5 μM and 100 μM to 195 nM, were ... | J Biol Chem 289: 21651-62 (2014) Article DOI: 10.1074/jbc.M114.574061 BindingDB Entry DOI: 10.7270/Q2BG2MVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM15794 ((1S,2R)-N-[1-(3,5-Difluoro-benzyl)-2-hydroxy-3-(S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | 4.8 | 37 |
Elan Pharmaceuticals | Assay Description Beta-cleavage ELISA assays were carried out in reaction buffer containing enzyme, substrate MBP-C125, and test compounds. Generated beta-cleaved prod... | J Med Chem 50: 776-81 (2007) Article DOI: 10.1021/jm061242y BindingDB Entry DOI: 10.7270/Q2K935TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phenylalanine--tRNA ligase alpha/beta subunit (Escherichia coli (Enterobacteria)) | BDBM231674 (N-(2-(1-(methylsulfonyl)cyclopropyl)ethyl)-2-(3-(5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | 8.0 | n/a |
AstraZeneca R&D Boston | Assay Description Compounds were solubilized in DMSO. Serial 2-fold dilutions covering two concentration ranges, 10 mM to 19.5 μM and 100 μM to 195 nM, were ... | J Biol Chem 289: 21651-62 (2014) Article DOI: 10.1074/jbc.M114.574061 BindingDB Entry DOI: 10.7270/Q2BG2MVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50210715 (CHEMBL230807 | N1-((2S,3R)-4-(3-methoxybenzylamino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 17: 3378-83 (2007) Article DOI: 10.1016/j.bmcl.2007.03.096 BindingDB Entry DOI: 10.7270/Q2RB748S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239369 (CHEMBL4100303) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of GAL4 DNA binding domain fused BCL6 BTB domain (unknown origin) expressed in HEK 293T/17 cells after 24 hrs by Bright-Glo luciferase cel... | J Med Chem 60: 4386-4402 (2017) Article DOI: 10.1021/acs.jmedchem.7b00359 BindingDB Entry DOI: 10.7270/Q2ZW1P1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phenylalanine--tRNA ligase alpha/beta subunit (Escherichia coli (Enterobacteria)) | BDBM231671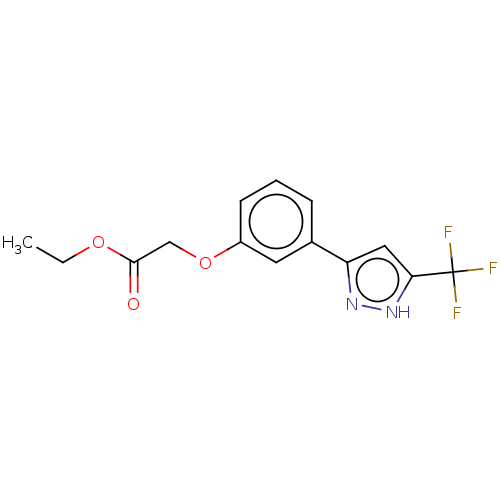 (Ethyl 2-(3-(5-(trifluoromethyl)-1H-pyrazol-3-yl)ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | 8.0 | n/a |
AstraZeneca R&D Boston | Assay Description Compounds were solubilized in DMSO. Serial 2-fold dilutions covering two concentration ranges, 10 mM to 19.5 μM and 100 μM to 195 nM, were ... | J Biol Chem 289: 21651-62 (2014) Article DOI: 10.1074/jbc.M114.574061 BindingDB Entry DOI: 10.7270/Q2BG2MVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239369 (CHEMBL4100303) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of GAL4 DNA binding domain fused BCL6 BTB domain (unknown origin) expressed in HEK 293T/17 cells after 24 hrs by Bright-Glo luciferase cel... | J Med Chem 60: 4386-4402 (2017) Article DOI: 10.1021/acs.jmedchem.7b00359 BindingDB Entry DOI: 10.7270/Q2ZW1P1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50210718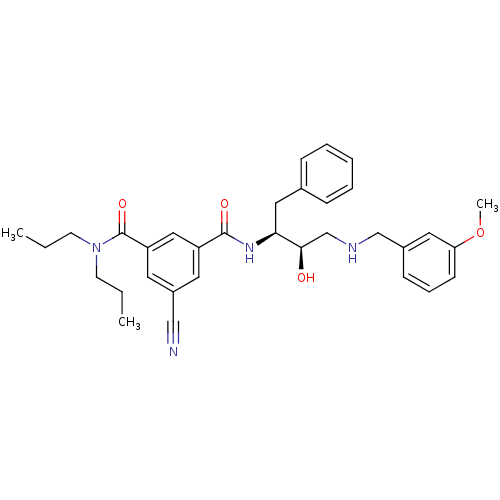 (CHEMBL231646 | N1-((2S,3R)-4-(3-methoxybenzylamino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 17: 3378-83 (2007) Article DOI: 10.1016/j.bmcl.2007.03.096 BindingDB Entry DOI: 10.7270/Q2RB748S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50491049 (CHEMBL2377573) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli str. K-12 substr. MG1655 LpxC using UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 20... | Bioorg Med Chem Lett 23: 2362-7 (2013) Article DOI: 10.1016/j.bmcl.2013.02.055 BindingDB Entry DOI: 10.7270/Q2F76GGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50210722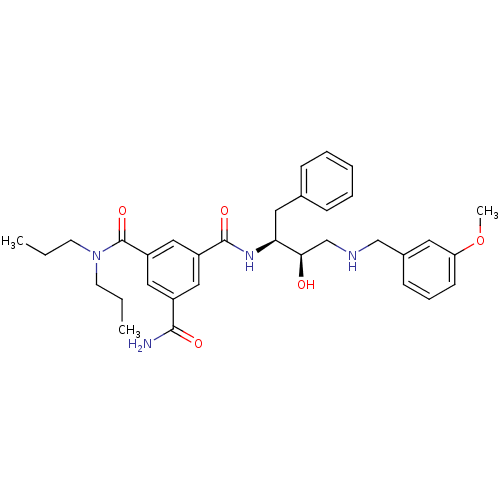 (CHEMBL231325 | N1-((2S,3R)-4-(3-methoxybenzylamino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 17: 3378-83 (2007) Article DOI: 10.1016/j.bmcl.2007.03.096 BindingDB Entry DOI: 10.7270/Q2RB748S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50210714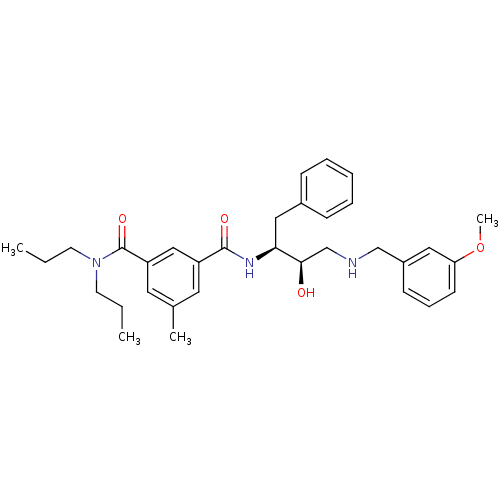 (CHEMBL231542 | N1-((2S,3R)-4-(3-methoxybenzylamino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 17: 3378-83 (2007) Article DOI: 10.1016/j.bmcl.2007.03.096 BindingDB Entry DOI: 10.7270/Q2RB748S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phenylalanine--tRNA ligase alpha/beta subunit (Escherichia coli (Enterobacteria)) | BDBM231676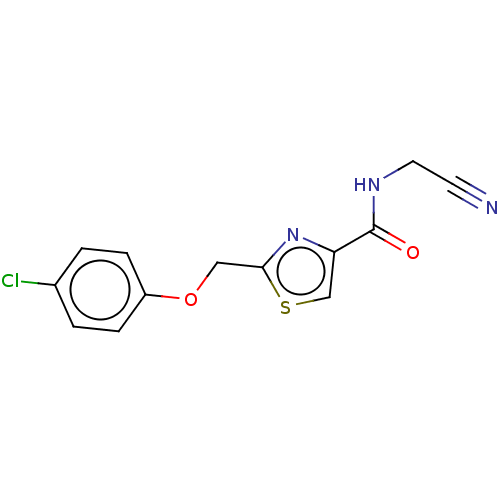 (2-((4-Chlorophenoxy)methyl)-N-(cyanomethyl)thiazol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | 8.0 | n/a |
AstraZeneca R&D Boston | Assay Description Compounds were solubilized in DMSO. Serial 2-fold dilutions covering two concentration ranges, 10 mM to 19.5 μM and 100 μM to 195 nM, were ... | J Biol Chem 289: 21651-62 (2014) Article DOI: 10.1074/jbc.M114.574061 BindingDB Entry DOI: 10.7270/Q2BG2MVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phenylalanine--tRNA ligase alpha/beta subunit (Escherichia coli (Enterobacteria)) | BDBM231669 (2-[3-[(4-Chloro-2-pyridyl)amino]phenoxy]-N-(1,1-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | 8.0 | n/a |
AstraZeneca R&D Boston | Assay Description Compounds were solubilized in DMSO. Serial 2-fold dilutions covering two concentration ranges, 10 mM to 19.5 μM and 100 μM to 195 nM, were ... | J Biol Chem 289: 21651-62 (2014) Article DOI: 10.1074/jbc.M114.574061 BindingDB Entry DOI: 10.7270/Q2BG2MVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239385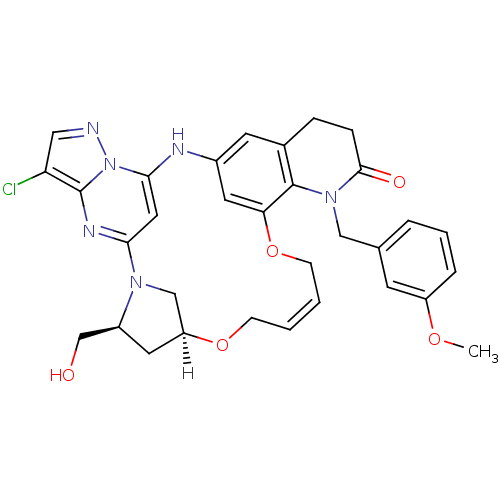 (CHEMBL4092565) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Binding affinity against alpha-1 adrenergic receptor in guinea pig cerebral cortical membranes by displacement of [3H]- WB-4101 | J Med Chem 60: 4386-4402 (2017) Article DOI: 10.1021/acs.jmedchem.7b00359 BindingDB Entry DOI: 10.7270/Q2ZW1P1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50491057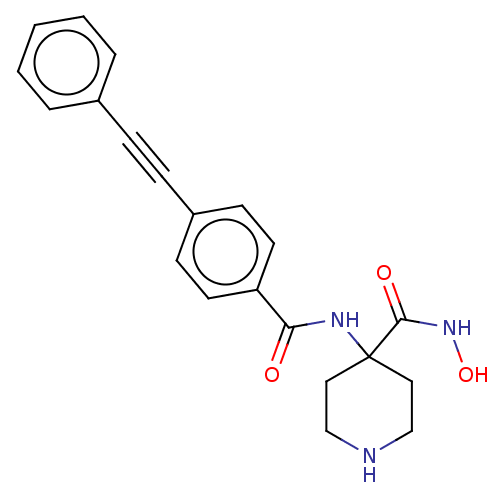 (CHEMBL2377691) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli str. K-12 substr. MG1655 LpxC using UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 20... | Bioorg Med Chem Lett 23: 2362-7 (2013) Article DOI: 10.1016/j.bmcl.2013.02.055 BindingDB Entry DOI: 10.7270/Q2F76GGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239368 (CHEMBL4073463) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of GAL4 DNA binding domain fused BCL6 BTB domain (unknown origin) expressed in HEK 293T/17 cells after 24 hrs by Bright-Glo luciferase cel... | J Med Chem 60: 4386-4402 (2017) Article DOI: 10.1021/acs.jmedchem.7b00359 BindingDB Entry DOI: 10.7270/Q2ZW1P1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM15796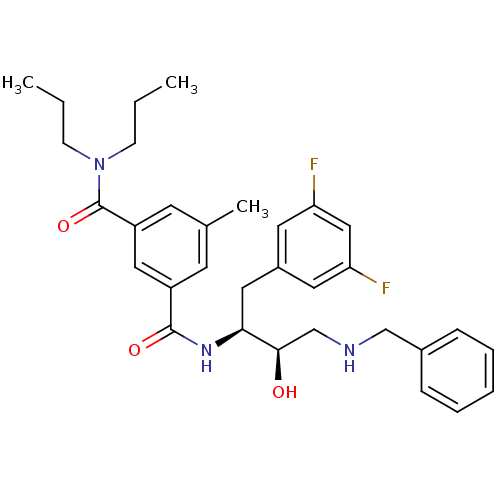 ((1S,2R)-N-[1-(3,5-Difluorobenzyl)-2-hydroxy-3-(ben...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | 4.8 | 37 |
Elan Pharmaceuticals | Assay Description Beta-cleavage ELISA assays were carried out in reaction buffer containing enzyme, substrate MBP-C125, and test compounds. Generated beta-cleaved prod... | J Med Chem 50: 776-81 (2007) Article DOI: 10.1021/jm061242y BindingDB Entry DOI: 10.7270/Q2K935TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM15792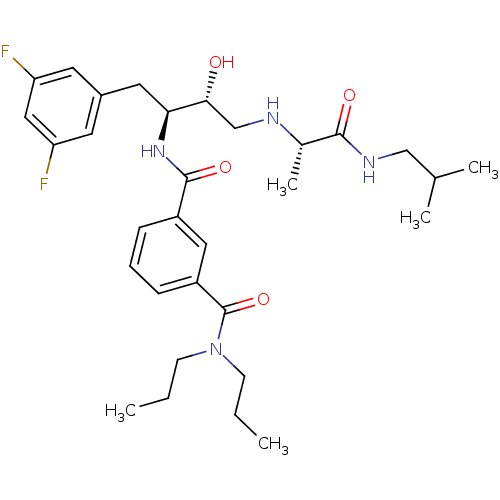 (3-N-[(2S,3R)-1-(3,5-difluorophenyl)-3-hydroxy-4-{[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | 4.8 | 37 |
Elan Pharmaceuticals | Assay Description Beta-cleavage ELISA assays were carried out in reaction buffer containing enzyme, substrate MBP-C125, and test compounds. Generated beta-cleaved prod... | J Med Chem 50: 776-81 (2007) Article DOI: 10.1021/jm061242y BindingDB Entry DOI: 10.7270/Q2K935TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50210713 (CHEMBL394596 | ethyl 3-(((2S,3R)-4-(3-methoxybenzy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 132 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 17: 3378-83 (2007) Article DOI: 10.1016/j.bmcl.2007.03.096 BindingDB Entry DOI: 10.7270/Q2RB748S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239368 (CHEMBL4073463) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of GAL4 DNA binding domain fused BCL6 BTB domain (unknown origin) expressed in HEK 293T/17 cells after 24 hrs by Bright-Glo luciferase cel... | J Med Chem 60: 4386-4402 (2017) Article DOI: 10.1021/acs.jmedchem.7b00359 BindingDB Entry DOI: 10.7270/Q2ZW1P1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50491060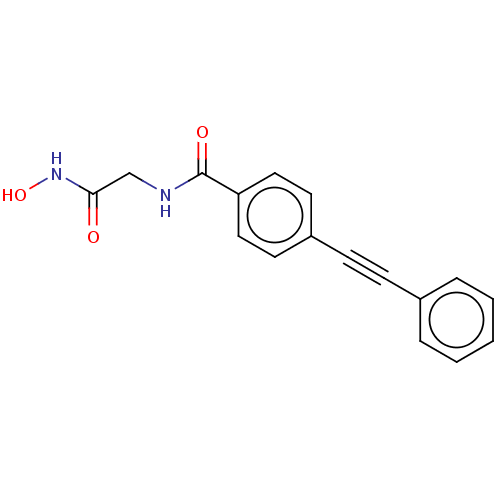 (CHEMBL2377695) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 148 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli str. K-12 substr. MG1655 LpxC using UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 20... | Bioorg Med Chem Lett 23: 2362-7 (2013) Article DOI: 10.1016/j.bmcl.2013.02.055 BindingDB Entry DOI: 10.7270/Q2F76GGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 152 total ) | Next | Last >> |