 Found 159 hits with Last Name = 'tocque' and Initial = 'b'
Found 159 hits with Last Name = 'tocque' and Initial = 'b' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50058202

((S)-2-[(2-{(S)-2-[((R)-2-Amino-3-mercapto-propiony...)Show SMILES CSCC[C@H](NC(=O)[C@@H]1Cc2ccccc2CN1C(=O)[C@H](C(C)C)N(C)C(=O)[C@@H](N)CS)C(O)=O Show InChI InChI=1S/C24H36N4O5S2/c1-14(2)20(27(3)22(30)17(25)13-34)23(31)28-12-16-8-6-5-7-15(16)11-19(28)21(29)26-18(24(32)33)9-10-35-4/h5-8,14,17-20,34H,9-13,25H2,1-4H3,(H,26,29)(H,32,33)/t17-,18-,19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Human farnesyl transferase |
Bioorg Med Chem Lett 5: 2677-2682 (1995)
Article DOI: 10.1016/0960-894X(95)00482-9
BindingDB Entry DOI: 10.7270/Q2QC03GF |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM50059852

((S)-2-((S)-2-((S)-2-((R)-2-amino-3-mercaptopropyla...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC[C@@H](NC[C@@H](N)CS)C(C)C)C(O)=O Show InChI InChI=1S/C22H38N4O3S2/c1-15(2)20(24-12-17(23)14-30)13-25-19(11-16-7-5-4-6-8-16)21(27)26-18(22(28)29)9-10-31-3/h4-8,15,17-20,24-25,30H,9-14,23H2,1-3H3,(H,26,27)(H,28,29)/t17-,18+,19+,20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibiion of bovine farnesyl transferase |
Bioorg Med Chem Lett 5: 2677-2682 (1995)
Article DOI: 10.1016/0960-894X(95)00482-9
BindingDB Entry DOI: 10.7270/Q2QC03GF |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM50284165
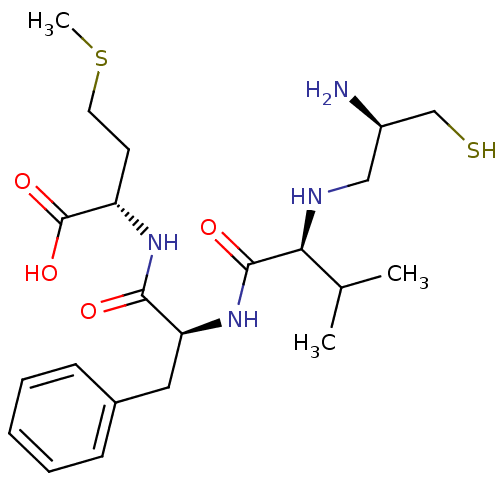
((S)-2-{(S)-2-[(S)-2-((R)-2-Amino-3-mercapto-propyl...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC[C@@H](N)CS)C(C)C)C(O)=O Show InChI InChI=1S/C22H36N4O4S2/c1-14(2)19(24-12-16(23)13-31)21(28)26-18(11-15-7-5-4-6-8-15)20(27)25-17(22(29)30)9-10-32-3/h4-8,14,16-19,24,31H,9-13,23H2,1-3H3,(H,25,27)(H,26,28)(H,29,30)/t16-,17+,18+,19+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of farnesyl transferase in NIH3T3 cell based assay under reducing (+DTT) conditions |
Bioorg Med Chem Lett 5: 2677-2682 (1995)
Article DOI: 10.1016/0960-894X(95)00482-9
BindingDB Entry DOI: 10.7270/Q2QC03GF |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50038199
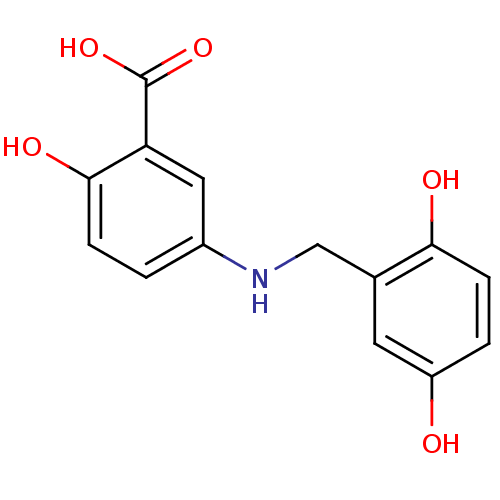
(5-(2,5-Dihydroxy-benzylamino)-2-hydroxy-benzoic ac...)Show InChI InChI=1S/C14H13NO5/c16-10-2-4-12(17)8(5-10)7-15-9-1-3-13(18)11(6-9)14(19)20/h1-6,15-18H,7H2,(H,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS
Curated by ChEMBL
| Assay Description
Inhibitory potency against protein tyrosine kinase activity associated with EGFR was evaluated using ER 22 cell membrane |
J Med Chem 37: 845-59 (1994)
BindingDB Entry DOI: 10.7270/Q2QV3KJ3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50453606

(CHEMBL168533)Show SMILES Oc1ccc(O)c(CNc2ccc(O)c(c2)C(=O)OCCCc2ccccc2)c1 Show InChI InChI=1S/C23H23NO5/c25-19-9-11-21(26)17(13-19)15-24-18-8-10-22(27)20(14-18)23(28)29-12-4-7-16-5-2-1-3-6-16/h1-3,5-6,8-11,13-14,24-27H,4,7,12,15H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS
Curated by ChEMBL
| Assay Description
Inhibitory potency against protein tyrosine kinase activity associated with EGFR was evaluated using ER 22 cell membrane |
J Med Chem 37: 845-59 (1994)
BindingDB Entry DOI: 10.7270/Q2QV3KJ3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50453600
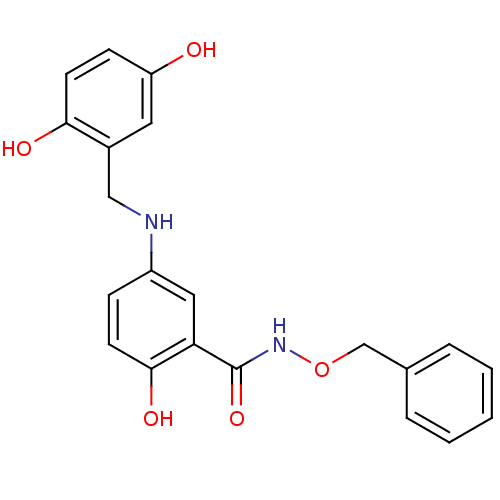
(CHEMBL355294)Show SMILES Oc1ccc(O)c(CNc2ccc(O)c(c2)C(=O)NOCc2ccccc2)c1 Show InChI InChI=1S/C21H20N2O5/c24-17-7-9-19(25)15(10-17)12-22-16-6-8-20(26)18(11-16)21(27)23-28-13-14-4-2-1-3-5-14/h1-11,22,24-26H,12-13H2,(H,23,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS
Curated by ChEMBL
| Assay Description
Inhibitory potency against protein tyrosine kinase activity associated with EGFR was evaluated using ER 22 cell membrane |
J Med Chem 37: 845-59 (1994)
BindingDB Entry DOI: 10.7270/Q2QV3KJ3 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50285838

(CHEMBL89836 | Pseudopeptide derivative)Show SMILES CSCC[C@H](NC(=O)[C@@H]1Cc2ccccc2CN1C(=O)CNCCSSCCNCC(=O)N1Cc2ccccc2C[C@H]1C(=O)N[C@@H](CCSC)C(O)=O)C(O)=O Show InChI InChI=1S/C38H52N6O8S4/c1-53-15-11-29(37(49)50)41-35(47)31-19-25-7-3-5-9-27(25)23-43(31)33(45)21-39-13-17-55-56-18-14-40-22-34(46)44-24-28-10-6-4-8-26(28)20-32(44)36(48)42-30(38(51)52)12-16-54-2/h3-10,29-32,39-40H,11-24H2,1-2H3,(H,41,47)(H,42,48)(H,49,50)(H,51,52)/t29-,30-,31-,32-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of farnesyl transferase |
Bioorg Med Chem Lett 5: 2677-2682 (1995)
Article DOI: 10.1016/0960-894X(95)00482-9
BindingDB Entry DOI: 10.7270/Q2QC03GF |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM13373

((2S)-2-[(2S)-2-[(2S)-2-[(2R)-2-amino-3-sulfanylpro...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@@H](N)CS)C(C)C)C(O)=O |r| Show InChI InChI=1S/C22H34N4O5S2/c1-13(2)18(26-19(27)15(23)12-32)21(29)25-17(11-14-7-5-4-6-8-14)20(28)24-16(22(30)31)9-10-33-3/h4-8,13,15-18,32H,9-12,23H2,1-3H3,(H,24,28)(H,25,29)(H,26,27)(H,30,31)/t15-,16-,17-,18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
Article
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibiion of bovine farnesyl transferase |
Bioorg Med Chem Lett 5: 2677-2682 (1995)
Article DOI: 10.1016/0960-894X(95)00482-9
BindingDB Entry DOI: 10.7270/Q2QC03GF |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM13373

((2S)-2-[(2S)-2-[(2S)-2-[(2R)-2-amino-3-sulfanylpro...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@@H](N)CS)C(C)C)C(O)=O |r| Show InChI InChI=1S/C22H34N4O5S2/c1-13(2)18(26-19(27)15(23)12-32)21(29)25-17(11-14-7-5-4-6-8-14)20(28)24-16(22(30)31)9-10-33-3/h4-8,13,15-18,32H,9-12,23H2,1-3H3,(H,24,28)(H,25,29)(H,26,27)(H,30,31)/t15-,16-,17-,18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
Article
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of farnesyl transferase in NIH3T3 cell based assay in non-reducing (-DTT) conditions |
Bioorg Med Chem Lett 5: 2677-2682 (1995)
Article DOI: 10.1016/0960-894X(95)00482-9
BindingDB Entry DOI: 10.7270/Q2QC03GF |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50453594
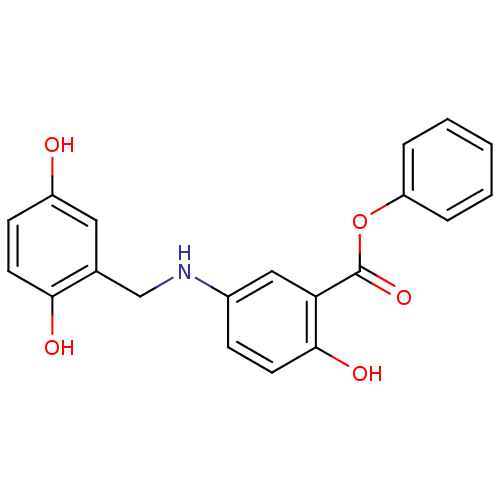
(CHEMBL169970)Show SMILES Oc1ccc(O)c(CNc2ccc(O)c(c2)C(=O)Oc2ccccc2)c1 Show InChI InChI=1S/C20H17NO5/c22-15-7-9-18(23)13(10-15)12-21-14-6-8-19(24)17(11-14)20(25)26-16-4-2-1-3-5-16/h1-11,21-24H,12H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS
Curated by ChEMBL
| Assay Description
Inhibitory potency against protein tyrosine kinase activity associated with EGFR was evaluated using ER 22 cell membrane |
J Med Chem 37: 845-59 (1994)
BindingDB Entry DOI: 10.7270/Q2QV3KJ3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50453576
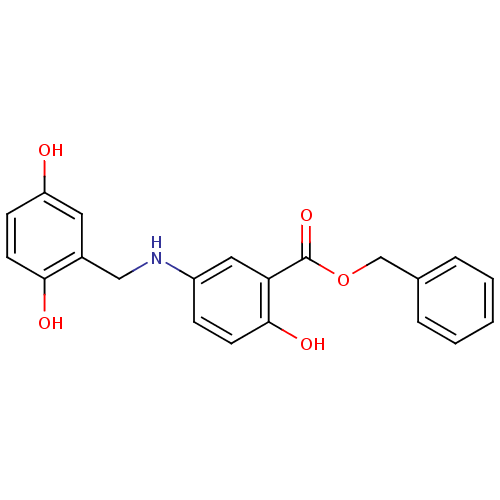
(CHEMBL352583)Show SMILES Oc1ccc(O)c(CNc2ccc(O)c(c2)C(=O)OCc2ccccc2)c1 Show InChI InChI=1S/C21H19NO5/c23-17-7-9-19(24)15(10-17)12-22-16-6-8-20(25)18(11-16)21(26)27-13-14-4-2-1-3-5-14/h1-11,22-25H,12-13H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS
Curated by ChEMBL
| Assay Description
Inhibitory potency against protein tyrosine kinase activity associated with EGFR was evaluated using ER 22 cell membrane |
J Med Chem 37: 845-59 (1994)
BindingDB Entry DOI: 10.7270/Q2QV3KJ3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50453601
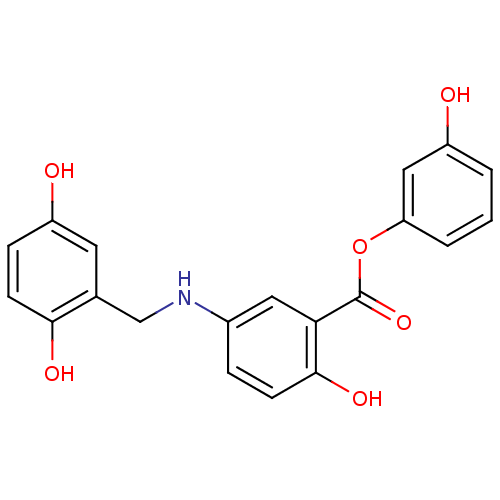
(CHEMBL422335)Show InChI InChI=1S/C20H17NO6/c22-14-2-1-3-16(10-14)27-20(26)17-9-13(4-6-19(17)25)21-11-12-8-15(23)5-7-18(12)24/h1-10,21-25H,11H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS
Curated by ChEMBL
| Assay Description
Inhibitory potency against protein tyrosine kinase activity associated with EGFR was evaluated using ER 22 cell membrane |
J Med Chem 37: 845-59 (1994)
BindingDB Entry DOI: 10.7270/Q2QV3KJ3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50453586
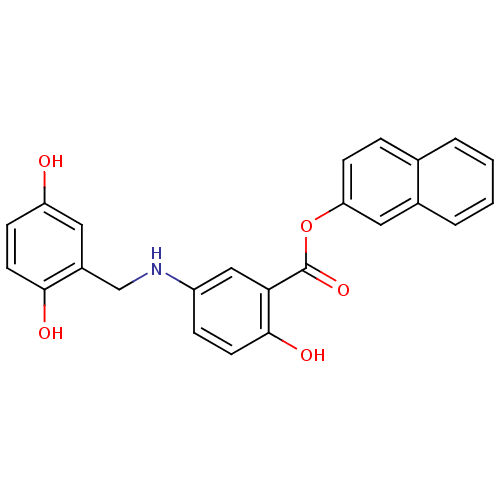
(CHEMBL169514)Show SMILES Oc1ccc(O)c(CNc2ccc(O)c(c2)C(=O)Oc2ccc3ccccc3c2)c1 Show InChI InChI=1S/C24H19NO5/c26-19-7-10-22(27)17(11-19)14-25-18-6-9-23(28)21(13-18)24(29)30-20-8-5-15-3-1-2-4-16(15)12-20/h1-13,25-28H,14H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS
Curated by ChEMBL
| Assay Description
Inhibitory potency against protein tyrosine kinase activity associated with EGFR was evaluated using ER 22 cell membrane |
J Med Chem 37: 845-59 (1994)
BindingDB Entry DOI: 10.7270/Q2QV3KJ3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50453584
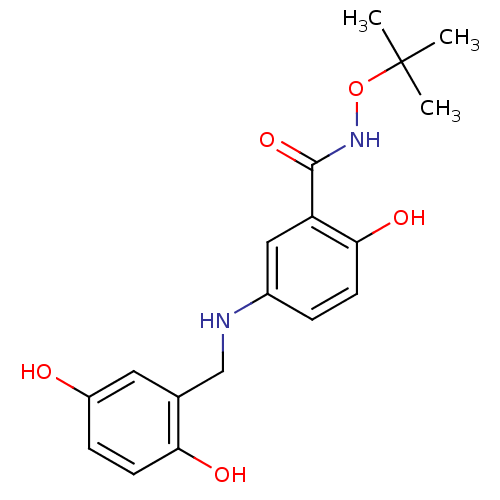
(CHEMBL170996)Show InChI InChI=1S/C18H22N2O5/c1-18(2,3)25-20-17(24)14-9-12(4-6-16(14)23)19-10-11-8-13(21)5-7-15(11)22/h4-9,19,21-23H,10H2,1-3H3,(H,20,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS
Curated by ChEMBL
| Assay Description
Inhibitory potency against protein tyrosine kinase activity associated with EGFR was evaluated using ER 22 cell membrane |
J Med Chem 37: 845-59 (1994)
BindingDB Entry DOI: 10.7270/Q2QV3KJ3 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50285846
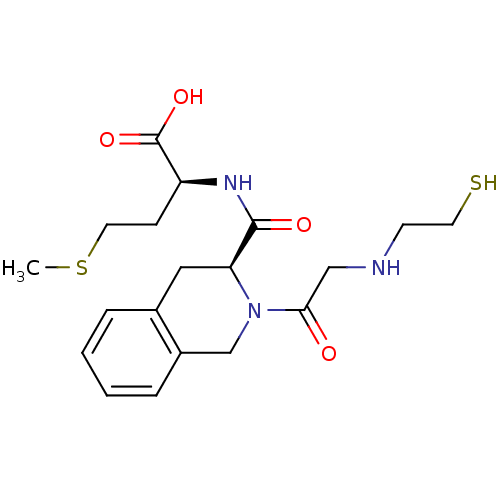
((S)-2-({(S)-2-[2-(2-Mercapto-ethylamino)-acetyl]-1...)Show SMILES CSCC[C@H](NC(=O)[C@@H]1Cc2ccccc2CN1C(=O)CNCCS)C(O)=O Show InChI InChI=1S/C19H27N3O4S2/c1-28-9-6-15(19(25)26)21-18(24)16-10-13-4-2-3-5-14(13)12-22(16)17(23)11-20-7-8-27/h2-5,15-16,20,27H,6-12H2,1H3,(H,21,24)(H,25,26)/t15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of farnesyl transferase |
Bioorg Med Chem Lett 5: 2677-2682 (1995)
Article DOI: 10.1016/0960-894X(95)00482-9
BindingDB Entry DOI: 10.7270/Q2QC03GF |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50453597
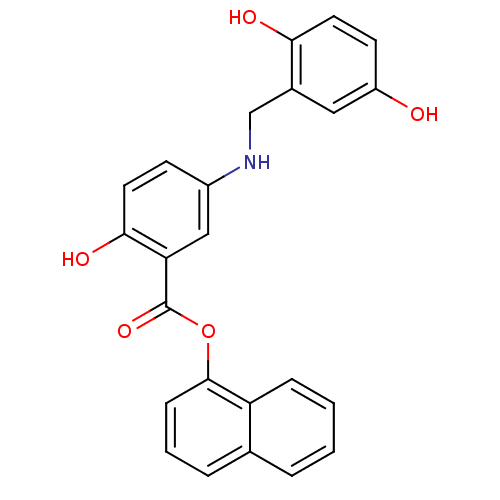
(CHEMBL168915)Show SMILES Oc1ccc(O)c(CNc2ccc(O)c(c2)C(=O)Oc2cccc3ccccc23)c1 Show InChI InChI=1S/C24H19NO5/c26-18-9-11-21(27)16(12-18)14-25-17-8-10-22(28)20(13-17)24(29)30-23-7-3-5-15-4-1-2-6-19(15)23/h1-13,25-28H,14H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS
Curated by ChEMBL
| Assay Description
Inhibitory potency against protein tyrosine kinase activity associated with EGFR was evaluated using ER 22 cell membrane |
J Med Chem 37: 845-59 (1994)
BindingDB Entry DOI: 10.7270/Q2QV3KJ3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50453607
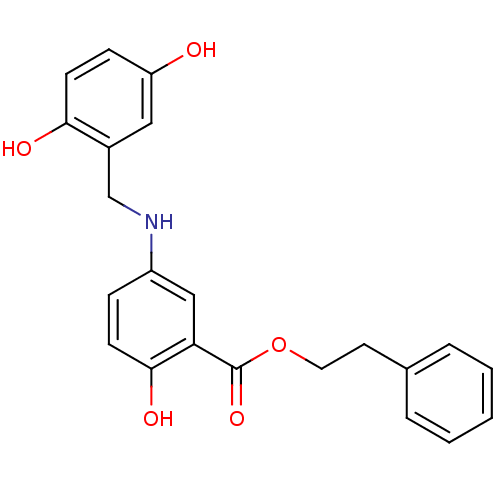
(CHEMBL168366)Show SMILES Oc1ccc(O)c(CNc2ccc(O)c(c2)C(=O)OCCc2ccccc2)c1 Show InChI InChI=1S/C22H21NO5/c24-18-7-9-20(25)16(12-18)14-23-17-6-8-21(26)19(13-17)22(27)28-11-10-15-4-2-1-3-5-15/h1-9,12-13,23-26H,10-11,14H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS
Curated by ChEMBL
| Assay Description
Inhibitory potency against protein tyrosine kinase activity associated with EGFR was evaluated using ER 22 cell membrane |
J Med Chem 37: 845-59 (1994)
BindingDB Entry DOI: 10.7270/Q2QV3KJ3 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50285842
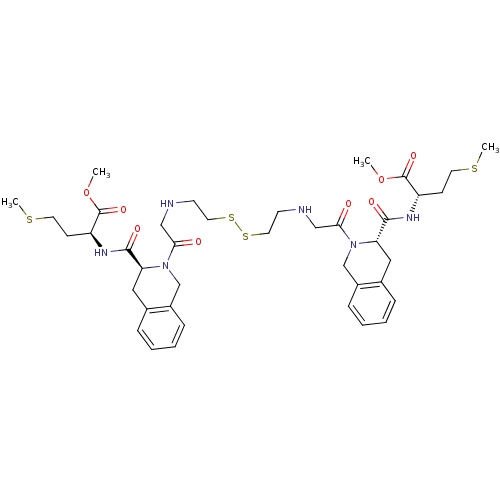
(CHEMBL420801 | Pseudopeptide derivative)Show SMILES COC(=O)[C@H](CCSC)NC(=O)[C@@H]1Cc2ccccc2CN1C(=O)CNCCSSCCNCC(=O)N1Cc2ccccc2C[C@H]1C(=O)N[C@@H](CCSC)C(=O)OC Show InChI InChI=1S/C40H56N6O8S4/c1-53-39(51)31(13-17-55-3)43-37(49)33-21-27-9-5-7-11-29(27)25-45(33)35(47)23-41-15-19-57-58-20-16-42-24-36(48)46-26-30-12-8-6-10-28(30)22-34(46)38(50)44-32(14-18-56-4)40(52)54-2/h5-12,31-34,41-42H,13-26H2,1-4H3,(H,43,49)(H,44,50)/t31-,32-,33-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 145 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of farnesyl transferase |
Bioorg Med Chem Lett 5: 2677-2682 (1995)
Article DOI: 10.1016/0960-894X(95)00482-9
BindingDB Entry DOI: 10.7270/Q2QC03GF |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50038199

(5-(2,5-Dihydroxy-benzylamino)-2-hydroxy-benzoic ac...)Show InChI InChI=1S/C14H13NO5/c16-10-2-4-12(17)8(5-10)7-15-9-1-3-13(18)11(6-9)14(19)20/h1-6,15-18H,7H2,(H,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS
Curated by ChEMBL
| Assay Description
Inhibition of EGFR(epidermal growth factor receptor) autophosphorylation in ER22 cell membranes |
J Med Chem 37: 845-59 (1994)
BindingDB Entry DOI: 10.7270/Q2QV3KJ3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50453607

(CHEMBL168366)Show SMILES Oc1ccc(O)c(CNc2ccc(O)c(c2)C(=O)OCCc2ccccc2)c1 Show InChI InChI=1S/C22H21NO5/c24-18-7-9-20(25)16(12-18)14-23-17-6-8-21(26)19(13-17)22(27)28-11-10-15-4-2-1-3-5-15/h1-9,12-13,23-26H,10-11,14H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS
Curated by ChEMBL
| Assay Description
Inhibition of EGFR(epidermal growth factor receptor) autophosphorylation in ER22 cell membranes |
J Med Chem 37: 845-59 (1994)
BindingDB Entry DOI: 10.7270/Q2QV3KJ3 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50285837
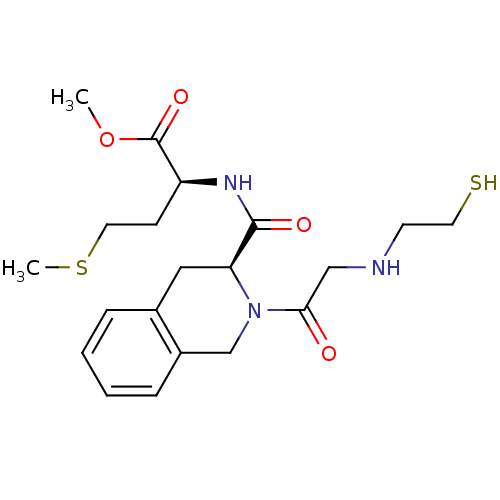
((S)-2-({(S)-2-[2-(2-Mercapto-ethylamino)-acetyl]-1...)Show SMILES COC(=O)[C@H](CCSC)NC(=O)[C@@H]1Cc2ccccc2CN1C(=O)CNCCS Show InChI InChI=1S/C20H29N3O4S2/c1-27-20(26)16(7-10-29-2)22-19(25)17-11-14-5-3-4-6-15(14)13-23(17)18(24)12-21-8-9-28/h3-6,16-17,21,28H,7-13H2,1-2H3,(H,22,25)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of farnesyl transferase |
Bioorg Med Chem Lett 5: 2677-2682 (1995)
Article DOI: 10.1016/0960-894X(95)00482-9
BindingDB Entry DOI: 10.7270/Q2QC03GF |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50453572
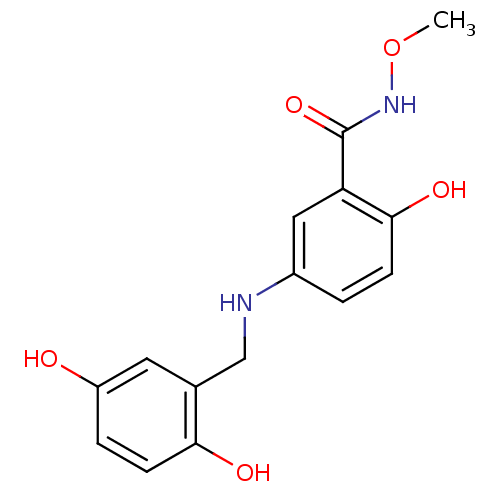
(CHEMBL169657)Show InChI InChI=1S/C15H16N2O5/c1-22-17-15(21)12-7-10(2-4-14(12)20)16-8-9-6-11(18)3-5-13(9)19/h2-7,16,18-20H,8H2,1H3,(H,17,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS
Curated by ChEMBL
| Assay Description
Inhibitory potency against protein tyrosine kinase activity associated with EGFR was evaluated using ER 22 cell membrane |
J Med Chem 37: 845-59 (1994)
BindingDB Entry DOI: 10.7270/Q2QV3KJ3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50453571
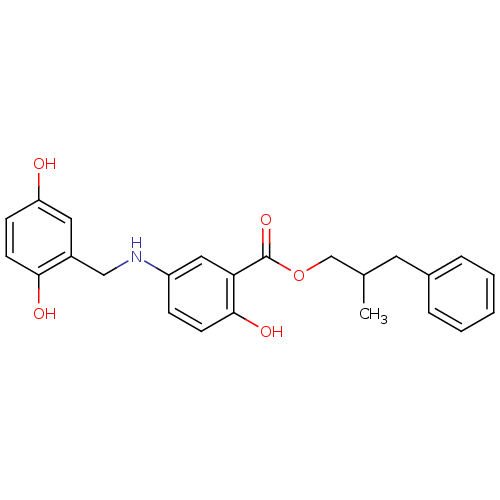
(CHEMBL171054)Show SMILES CC(COC(=O)c1cc(NCc2cc(O)ccc2O)ccc1O)Cc1ccccc1 Show InChI InChI=1S/C24H25NO5/c1-16(11-17-5-3-2-4-6-17)15-30-24(29)21-13-19(7-9-23(21)28)25-14-18-12-20(26)8-10-22(18)27/h2-10,12-13,16,25-28H,11,14-15H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS
Curated by ChEMBL
| Assay Description
Inhibitory potency against protein tyrosine kinase activity associated with EGFR was evaluated using ER 22 cell membrane |
J Med Chem 37: 845-59 (1994)
BindingDB Entry DOI: 10.7270/Q2QV3KJ3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50453603
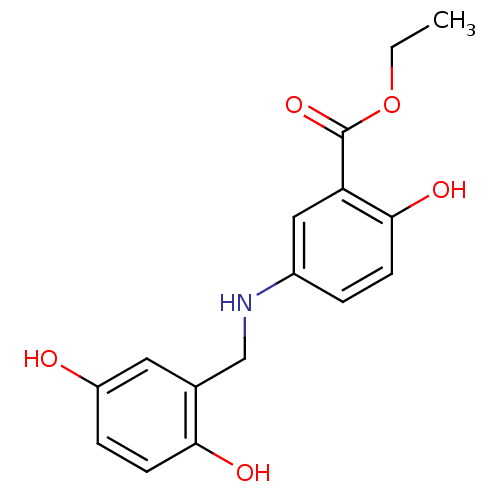
(CHEMBL355857)Show InChI InChI=1S/C16H17NO5/c1-2-22-16(21)13-8-11(3-5-15(13)20)17-9-10-7-12(18)4-6-14(10)19/h3-8,17-20H,2,9H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS
Curated by ChEMBL
| Assay Description
Inhibitory potency against protein tyrosine kinase activity associated with EGFR was evaluated using ER 22 cell membrane |
J Med Chem 37: 845-59 (1994)
BindingDB Entry DOI: 10.7270/Q2QV3KJ3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50453605
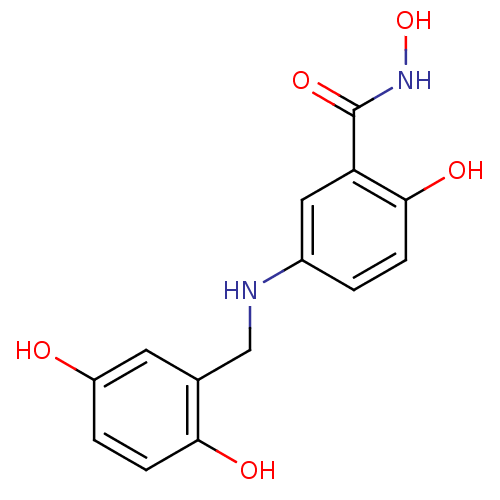
(CHEMBL172449)Show InChI InChI=1S/C14H14N2O5/c17-10-2-4-12(18)8(5-10)7-15-9-1-3-13(19)11(6-9)14(20)16-21/h1-6,15,17-19,21H,7H2,(H,16,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS
Curated by ChEMBL
| Assay Description
Inhibitory potency against protein tyrosine kinase activity associated with EGFR was evaluated using ER 22 cell membrane |
J Med Chem 37: 845-59 (1994)
BindingDB Entry DOI: 10.7270/Q2QV3KJ3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50453577
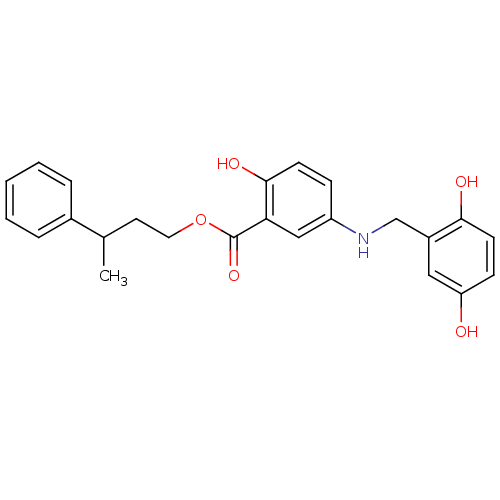
(CHEMBL354128)Show SMILES CC(CCOC(=O)c1cc(NCc2cc(O)ccc2O)ccc1O)c1ccccc1 Show InChI InChI=1S/C24H25NO5/c1-16(17-5-3-2-4-6-17)11-12-30-24(29)21-14-19(7-9-23(21)28)25-15-18-13-20(26)8-10-22(18)27/h2-10,13-14,16,25-28H,11-12,15H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS
Curated by ChEMBL
| Assay Description
Inhibitory potency against protein tyrosine kinase activity associated with EGFR was evaluated using ER 22 cell membrane |
J Med Chem 37: 845-59 (1994)
BindingDB Entry DOI: 10.7270/Q2QV3KJ3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50453569
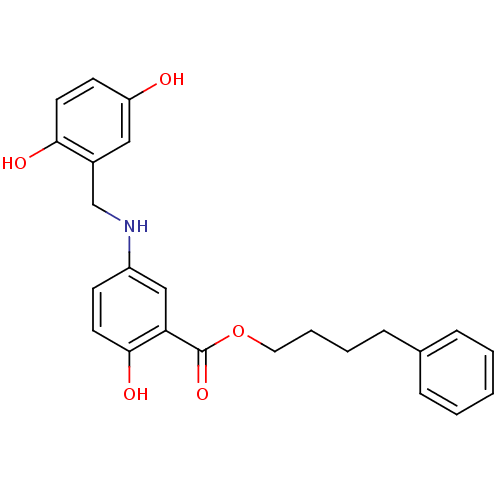
(CHEMBL435902)Show SMILES Oc1ccc(O)c(CNc2ccc(O)c(c2)C(=O)OCCCCc2ccccc2)c1 Show InChI InChI=1S/C24H25NO5/c26-20-10-12-22(27)18(14-20)16-25-19-9-11-23(28)21(15-19)24(29)30-13-5-4-8-17-6-2-1-3-7-17/h1-3,6-7,9-12,14-15,25-28H,4-5,8,13,16H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS
Curated by ChEMBL
| Assay Description
Inhibitory potency against protein tyrosine kinase activity associated with EGFR was evaluated using ER 22 cell membrane |
J Med Chem 37: 845-59 (1994)
BindingDB Entry DOI: 10.7270/Q2QV3KJ3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50038205

(5-(2,5-Dihydroxy-benzylamino)-2-hydroxy-benzoic ac...)Show InChI InChI=1S/C15H15NO5/c1-21-15(20)12-7-10(2-4-14(12)19)16-8-9-6-11(17)3-5-13(9)18/h2-7,16-19H,8H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS
Curated by ChEMBL
| Assay Description
Inhibitory potency against protein tyrosine kinase activity associated with EGFR was evaluated using ER 22 cell membrane |
J Med Chem 37: 845-59 (1994)
BindingDB Entry DOI: 10.7270/Q2QV3KJ3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50453606

(CHEMBL168533)Show SMILES Oc1ccc(O)c(CNc2ccc(O)c(c2)C(=O)OCCCc2ccccc2)c1 Show InChI InChI=1S/C23H23NO5/c25-19-9-11-21(26)17(13-19)15-24-18-8-10-22(27)20(14-18)23(28)29-12-4-7-16-5-2-1-3-6-16/h1-3,5-6,8-11,13-14,24-27H,4,7,12,15H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS
Curated by ChEMBL
| Assay Description
Inhibition of EGFR(epidermal growth factor receptor) autophosphorylation in ER22 cell membranes |
J Med Chem 37: 845-59 (1994)
BindingDB Entry DOI: 10.7270/Q2QV3KJ3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50453604
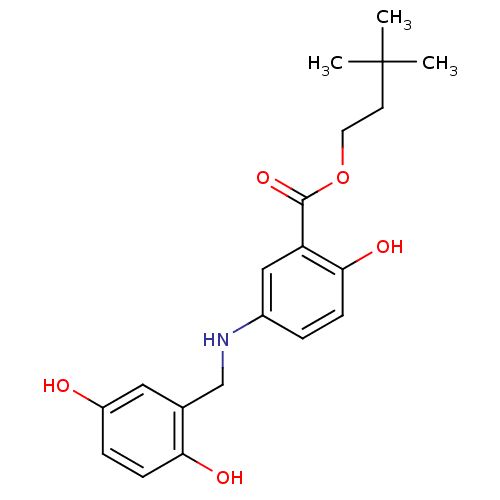
(CHEMBL353028)Show InChI InChI=1S/C20H25NO5/c1-20(2,3)8-9-26-19(25)16-11-14(4-6-18(16)24)21-12-13-10-15(22)5-7-17(13)23/h4-7,10-11,21-24H,8-9,12H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS
Curated by ChEMBL
| Assay Description
Inhibitory potency against protein tyrosine kinase activity associated with EGFR was evaluated using ER 22 cell membrane |
J Med Chem 37: 845-59 (1994)
BindingDB Entry DOI: 10.7270/Q2QV3KJ3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50453578

(CHEMBL368296)Show SMILES CC(CCOC(=O)c1cc(NCc2cc(O)ccc2O)ccc1O)CC(C)(C)C Show InChI InChI=1S/C23H31NO5/c1-15(13-23(2,3)4)9-10-29-22(28)19-12-17(5-7-21(19)27)24-14-16-11-18(25)6-8-20(16)26/h5-8,11-12,15,24-27H,9-10,13-14H2,1-4H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS
Curated by ChEMBL
| Assay Description
Inhibition of EGF-stimulated DNA synthesis in ER22 cells, by measuring [3H]Me-dT incorporation into ER 22 cells |
J Med Chem 37: 845-59 (1994)
BindingDB Entry DOI: 10.7270/Q2QV3KJ3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50453581

(CHEMBL355853)Show SMILES CC(COC(=O)c1cc(NCc2cc(O)ccc2O)ccc1O)CC(C)(C)C Show InChI InChI=1S/C22H29NO5/c1-14(11-22(2,3)4)13-28-21(27)18-10-16(5-7-20(18)26)23-12-15-9-17(24)6-8-19(15)25/h5-10,14,23-26H,11-13H2,1-4H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS
Curated by ChEMBL
| Assay Description
Inhibition of EGF-stimulated DNA synthesis in ER22 cells, by measuring [3H]Me-dT incorporation into ER 22 cells |
J Med Chem 37: 845-59 (1994)
BindingDB Entry DOI: 10.7270/Q2QV3KJ3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50453583
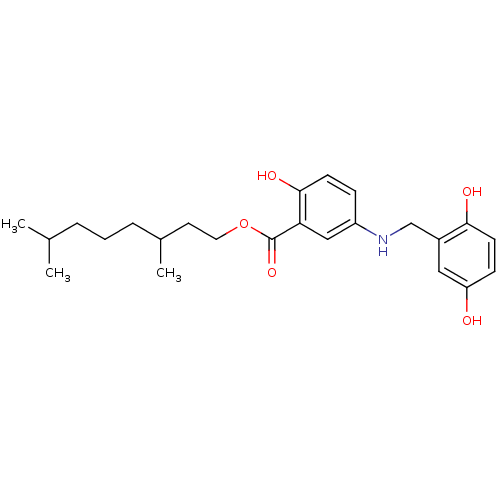
(CHEMBL170997)Show SMILES CC(C)CCCC(C)CCOC(=O)c1cc(NCc2cc(O)ccc2O)ccc1O Show InChI InChI=1S/C24H33NO5/c1-16(2)5-4-6-17(3)11-12-30-24(29)21-14-19(7-9-23(21)28)25-15-18-13-20(26)8-10-22(18)27/h7-10,13-14,16-17,25-28H,4-6,11-12,15H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS
Curated by ChEMBL
| Assay Description
Inhibition of EGF-stimulated DNA synthesis in ER22 cells, by measuring [3H]Me-dT incorporation into ER 22 cells |
J Med Chem 37: 845-59 (1994)
BindingDB Entry DOI: 10.7270/Q2QV3KJ3 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM13373

((2S)-2-[(2S)-2-[(2S)-2-[(2R)-2-amino-3-sulfanylpro...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@@H](N)CS)C(C)C)C(O)=O |r| Show InChI InChI=1S/C22H34N4O5S2/c1-13(2)18(26-19(27)15(23)12-32)21(29)25-17(11-14-7-5-4-6-8-14)20(28)24-16(22(30)31)9-10-33-3/h4-8,13,15-18,32H,9-12,23H2,1-3H3,(H,24,28)(H,25,29)(H,26,27)(H,30,31)/t15-,16-,17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
Article
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Human farnesyl transferase |
Bioorg Med Chem Lett 5: 2677-2682 (1995)
Article DOI: 10.1016/0960-894X(95)00482-9
BindingDB Entry DOI: 10.7270/Q2QC03GF |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029728

(2'-Hydroxy-[1,1';4',1'']terphenyl-4-carboxylic aci...)Show SMILES Oc1cc(ccc1-c1ccc(cc1)C(=O)NCc1ccccc1)-c1ccccc1 Show InChI InChI=1S/C26H21NO2/c28-25-17-23(20-9-5-2-6-10-20)15-16-24(25)21-11-13-22(14-12-21)26(29)27-18-19-7-3-1-4-8-19/h1-17,28H,18H2,(H,27,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
In vitro inhibition of intrinsic tyrosine protein kinase activity of the Epidermal growth factor receptor using tripeptide RR-Src |
J Med Chem 38: 4693-703 (1995)
BindingDB Entry DOI: 10.7270/Q2FX78G6 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029741
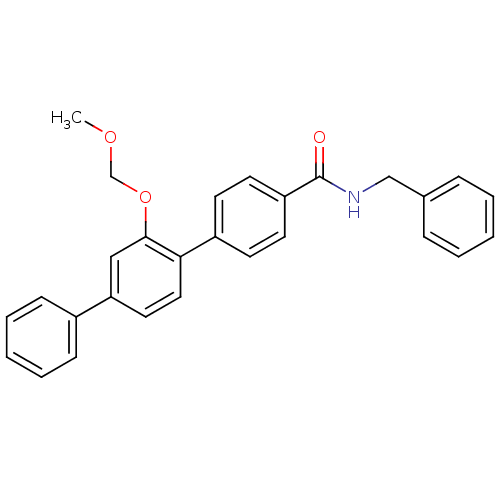
(2'-Methoxymethoxy-[1,1';4',1'']terphenyl-4-carboxy...)Show SMILES COCOc1cc(ccc1-c1ccc(cc1)C(=O)NCc1ccccc1)-c1ccccc1 Show InChI InChI=1S/C28H25NO3/c1-31-20-32-27-18-25(22-10-6-3-7-11-22)16-17-26(27)23-12-14-24(15-13-23)28(30)29-19-21-8-4-2-5-9-21/h2-18H,19-20H2,1H3,(H,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
In vitro inhibition of intrinsic tyrosine protein kinase activity of the Epidermal growth factor receptor using tripeptide RR-Src |
J Med Chem 38: 4693-703 (1995)
BindingDB Entry DOI: 10.7270/Q2FX78G6 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50038205

(5-(2,5-Dihydroxy-benzylamino)-2-hydroxy-benzoic ac...)Show InChI InChI=1S/C15H15NO5/c1-21-15(20)12-7-10(2-4-14(12)19)16-8-9-6-11(17)3-5-13(9)18/h2-7,16-19H,8H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS
Curated by ChEMBL
| Assay Description
Inhibition of EGFR(epidermal growth factor receptor) autophosphorylation in ER22 cell membranes |
J Med Chem 37: 845-59 (1994)
BindingDB Entry DOI: 10.7270/Q2QV3KJ3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50453596

(CHEMBL435662)Show SMILES CC(C)c1ccc(COC(=O)c2cc(NCc3cc(O)ccc3O)ccc2O)cc1 Show InChI InChI=1S/C24H25NO5/c1-15(2)17-5-3-16(4-6-17)14-30-24(29)21-12-19(7-9-23(21)28)25-13-18-11-20(26)8-10-22(18)27/h3-12,15,25-28H,13-14H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS
Curated by ChEMBL
| Assay Description
Inhibitory potency against protein tyrosine kinase activity associated with EGFR was evaluated using ER 22 cell membrane |
J Med Chem 37: 845-59 (1994)
BindingDB Entry DOI: 10.7270/Q2QV3KJ3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50453602

(CHEMBL170111)Show InChI InChI=1S/C18H21NO5/c1-18(2,3)24-17(23)14-9-12(4-6-16(14)22)19-10-11-8-13(20)5-7-15(11)21/h4-9,19-22H,10H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS
Curated by ChEMBL
| Assay Description
Inhibitory potency against protein tyrosine kinase activity associated with EGFR was evaluated using ER 22 cell membrane |
J Med Chem 37: 845-59 (1994)
BindingDB Entry DOI: 10.7270/Q2QV3KJ3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50453589

(CHEMBL352589)Show SMILES Oc1ccc(O)c(CNc2ccc(O)c(CC(=O)NOCc3ccccc3)c2)c1 Show InChI InChI=1S/C22H22N2O5/c25-19-7-9-21(27)17(11-19)13-23-18-6-8-20(26)16(10-18)12-22(28)24-29-14-15-4-2-1-3-5-15/h1-11,23,25-27H,12-14H2,(H,24,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS
Curated by ChEMBL
| Assay Description
Inhibitory potency against protein tyrosine kinase activity associated with EGFR was evaluated using ER 22 cell membrane |
J Med Chem 37: 845-59 (1994)
BindingDB Entry DOI: 10.7270/Q2QV3KJ3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029741

(2'-Methoxymethoxy-[1,1';4',1'']terphenyl-4-carboxy...)Show SMILES COCOc1cc(ccc1-c1ccc(cc1)C(=O)NCc1ccccc1)-c1ccccc1 Show InChI InChI=1S/C28H25NO3/c1-31-20-32-27-18-25(22-10-6-3-7-11-22)16-17-26(27)23-12-14-24(15-13-23)28(30)29-19-21-8-4-2-5-9-21/h2-18H,19-20H2,1H3,(H,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
In vitro inhibition of EGF-stimulated DNA synthesis of ER 22 cells by following the incorporation of methyl-[3H]thymidine. |
J Med Chem 38: 4693-703 (1995)
BindingDB Entry DOI: 10.7270/Q2FX78G6 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50453575

(CHEMBL168363)Show SMILES Oc1ccc(O)c(CNc2ccc(O)c(c2)C(=O)OCC2CCCCC2)c1 Show InChI InChI=1S/C21H25NO5/c23-17-7-9-19(24)15(10-17)12-22-16-6-8-20(25)18(11-16)21(26)27-13-14-4-2-1-3-5-14/h6-11,14,22-25H,1-5,12-13H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS
Curated by ChEMBL
| Assay Description
Inhibitory potency against protein tyrosine kinase activity associated with EGFR was evaluated using ER 22 cell membrane |
J Med Chem 37: 845-59 (1994)
BindingDB Entry DOI: 10.7270/Q2QV3KJ3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50453569

(CHEMBL435902)Show SMILES Oc1ccc(O)c(CNc2ccc(O)c(c2)C(=O)OCCCCc2ccccc2)c1 Show InChI InChI=1S/C24H25NO5/c26-20-10-12-22(27)18(14-20)16-25-19-9-11-23(28)21(15-19)24(29)30-13-5-4-8-17-6-2-1-3-7-17/h1-3,6-7,9-12,14-15,25-28H,4-5,8,13,16H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS
Curated by ChEMBL
| Assay Description
Inhibition of EGF-stimulated DNA synthesis in ER22 cells, by measuring [3H]Me-dT incorporation into ER 22 cells |
J Med Chem 37: 845-59 (1994)
BindingDB Entry DOI: 10.7270/Q2QV3KJ3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50453568

(CHEMBL169885)Show SMILES [H][C@]12C[C@]3([H])C[C@]([H])(C1)CC(CCOC(=O)c1cc(NCc4cc(O)ccc4O)ccc1O)(C2)C3 |TLB:8:6:34:1.33.2,8:1:34:6.9.5,THB:2:3:9:1.33.8,2:1:9:3.34.5| Show InChI InChI=1S/C26H31NO5/c28-21-2-4-23(29)19(10-21)15-27-20-1-3-24(30)22(11-20)25(31)32-6-5-26-12-16-7-17(13-26)9-18(8-16)14-26/h1-4,10-11,16-18,27-30H,5-9,12-15H2/t16-,17+,18-,26? | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS
Curated by ChEMBL
| Assay Description
Inhibition of EGF-stimulated DNA synthesis in ER22 cells, by measuring [3H]Me-dT incorporation into ER 22 cells |
J Med Chem 37: 845-59 (1994)
BindingDB Entry DOI: 10.7270/Q2QV3KJ3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50453595
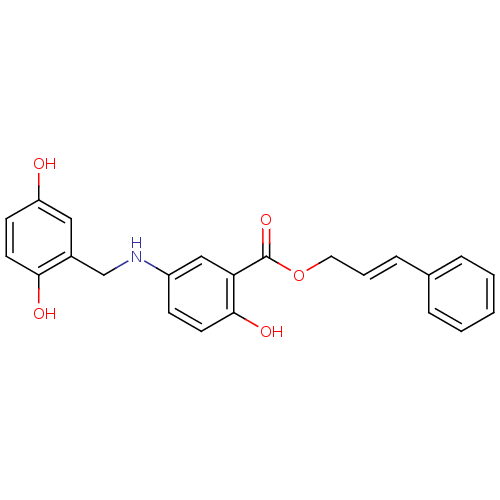
(CHEMBL172333)Show SMILES Oc1ccc(O)c(CNc2ccc(O)c(c2)C(=O)OC\C=C\c2ccccc2)c1 Show InChI InChI=1S/C23H21NO5/c25-19-9-11-21(26)17(13-19)15-24-18-8-10-22(27)20(14-18)23(28)29-12-4-7-16-5-2-1-3-6-16/h1-11,13-14,24-27H,12,15H2/b7-4+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS
Curated by ChEMBL
| Assay Description
Inhibitory potency against protein tyrosine kinase activity associated with EGFR was evaluated using ER 22 cell membrane |
J Med Chem 37: 845-59 (1994)
BindingDB Entry DOI: 10.7270/Q2QV3KJ3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50453596

(CHEMBL435662)Show SMILES CC(C)c1ccc(COC(=O)c2cc(NCc3cc(O)ccc3O)ccc2O)cc1 Show InChI InChI=1S/C24H25NO5/c1-15(2)17-5-3-16(4-6-17)14-30-24(29)21-12-19(7-9-23(21)28)25-13-18-11-20(26)8-10-22(18)27/h3-12,15,25-28H,13-14H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS
Curated by ChEMBL
| Assay Description
Inhibition of EGF-stimulated DNA synthesis in ER22 cells, by measuring [3H]Me-dT incorporation into ER 22 cells |
J Med Chem 37: 845-59 (1994)
BindingDB Entry DOI: 10.7270/Q2QV3KJ3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029728

(2'-Hydroxy-[1,1';4',1'']terphenyl-4-carboxylic aci...)Show SMILES Oc1cc(ccc1-c1ccc(cc1)C(=O)NCc1ccccc1)-c1ccccc1 Show InChI InChI=1S/C26H21NO2/c28-25-17-23(20-9-5-2-6-10-20)15-16-24(25)21-11-13-22(14-12-21)26(29)27-18-19-7-3-1-4-8-19/h1-17,28H,18H2,(H,27,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
In vitro inhibition of EGF-stimulated DNA synthesis of ER 22 cells by following the incorporation of methyl-[3H]thymidine. |
J Med Chem 38: 4693-703 (1995)
BindingDB Entry DOI: 10.7270/Q2FX78G6 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50453606

(CHEMBL168533)Show SMILES Oc1ccc(O)c(CNc2ccc(O)c(c2)C(=O)OCCCc2ccccc2)c1 Show InChI InChI=1S/C23H23NO5/c25-19-9-11-21(26)17(13-19)15-24-18-8-10-22(27)20(14-18)23(28)29-12-4-7-16-5-2-1-3-6-16/h1-3,5-6,8-11,13-14,24-27H,4,7,12,15H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS
Curated by ChEMBL
| Assay Description
Inhibition of EGF-stimulated DNA synthesis in ER22 cells, by measuring [3H]Me-dT incorporation into ER 22 cells |
J Med Chem 37: 845-59 (1994)
BindingDB Entry DOI: 10.7270/Q2QV3KJ3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50453592
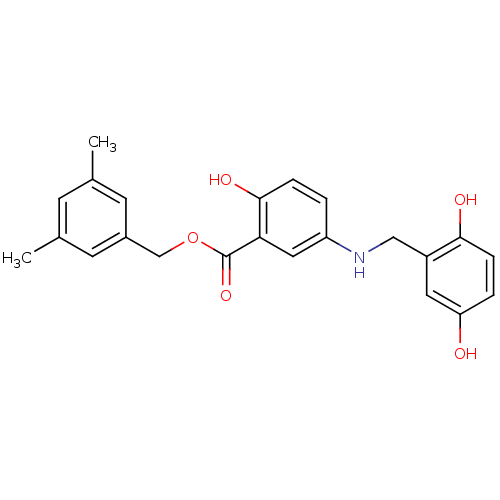
(CHEMBL169356)Show SMILES Cc1cc(C)cc(COC(=O)c2cc(NCc3cc(O)ccc3O)ccc2O)c1 Show InChI InChI=1S/C23H23NO5/c1-14-7-15(2)9-16(8-14)13-29-23(28)20-11-18(3-5-22(20)27)24-12-17-10-19(25)4-6-21(17)26/h3-11,24-27H,12-13H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS
Curated by ChEMBL
| Assay Description
Inhibition of EGF-stimulated DNA synthesis in ER22 cells, by measuring [3H]Me-dT incorporation into ER 22 cells |
J Med Chem 37: 845-59 (1994)
BindingDB Entry DOI: 10.7270/Q2QV3KJ3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50453595

(CHEMBL172333)Show SMILES Oc1ccc(O)c(CNc2ccc(O)c(c2)C(=O)OC\C=C\c2ccccc2)c1 Show InChI InChI=1S/C23H21NO5/c25-19-9-11-21(26)17(13-19)15-24-18-8-10-22(27)20(14-18)23(28)29-12-4-7-16-5-2-1-3-6-16/h1-11,13-14,24-27H,12,15H2/b7-4+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS
Curated by ChEMBL
| Assay Description
Inhibition of EGF-stimulated DNA synthesis in ER22 cells, by measuring [3H]Me-dT incorporation into ER 22 cells |
J Med Chem 37: 845-59 (1994)
BindingDB Entry DOI: 10.7270/Q2QV3KJ3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data