 Found 105 hits with Last Name = 'stewart' and Initial = 'bh'
Found 105 hits with Last Name = 'stewart' and Initial = 'bh' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50290981
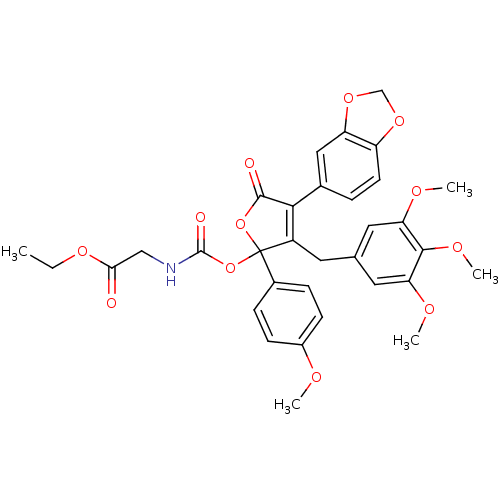
(CHEMBL319452 | PD-163140 | [4-Benzo[1,3]dioxol-5-y...)Show SMILES CCOC(=O)CNC(=O)OC1(OC(=O)C(=C1Cc1cc(OC)c(OC)c(OC)c1)c1ccc2OCOc2c1)c1ccc(OC)cc1 |c:14| Show InChI InChI=1S/C33H33NO12/c1-6-42-28(35)17-34-32(37)46-33(21-8-10-22(38-2)11-9-21)23(13-19-14-26(39-3)30(41-5)27(15-19)40-4)29(31(36)45-33)20-7-12-24-25(16-20)44-18-43-24/h7-12,14-16H,6,13,17-18H2,1-5H3,(H,34,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for human Endothelin A receptor expressed in LtK- cells |
Bioorg Med Chem Lett 7: 297-302 (1997)
Article DOI: 10.1016/S0960-894X(97)00002-4
BindingDB Entry DOI: 10.7270/Q2B8584G |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50034267
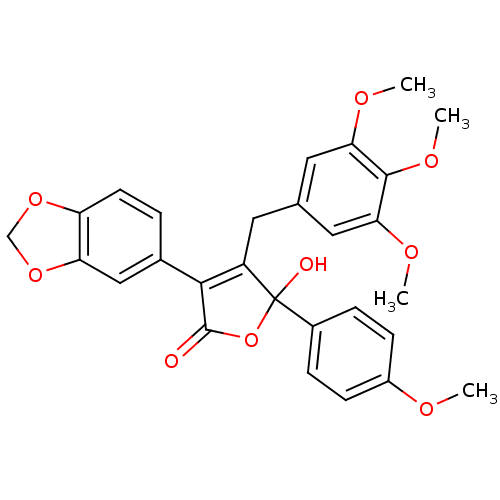
(3-(benzo[d][1,3]dioxol-5-yl)-5-hydroxy-5-(4-methox...)Show SMILES COc1ccc(cc1)C1(O)OC(=O)C(=C1Cc1cc(OC)c(OC)c(OC)c1)c1ccc2OCOc2c1 |c:14| Show InChI InChI=1S/C28H26O9/c1-31-19-8-6-18(7-9-19)28(30)20(11-16-12-23(32-2)26(34-4)24(13-16)33-3)25(27(29)37-28)17-5-10-21-22(14-17)36-15-35-21/h5-10,12-14,30H,11,15H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for human Endothelin A receptor expressed in LtK- cells |
Bioorg Med Chem Lett 7: 297-302 (1997)
Article DOI: 10.1016/S0960-894X(97)00002-4
BindingDB Entry DOI: 10.7270/Q2B8584G |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50290983
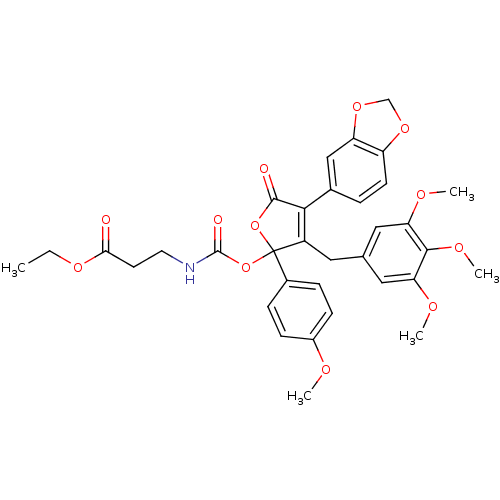
(3-[4-Benzo[1,3]dioxol-5-yl-2-(4-methoxy-phenyl)-5-...)Show SMILES CCOC(=O)CCNC(=O)OC1(OC(=O)C(=C1Cc1cc(OC)c(OC)c(OC)c1)c1ccc2OCOc2c1)c1ccc(OC)cc1 |c:15| Show InChI InChI=1S/C34H35NO12/c1-6-43-29(36)13-14-35-33(38)47-34(22-8-10-23(39-2)11-9-22)24(15-20-16-27(40-3)31(42-5)28(17-20)41-4)30(32(37)46-34)21-7-12-25-26(18-21)45-19-44-25/h7-12,16-18H,6,13-15,19H2,1-5H3,(H,35,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for human Endothelin A receptor expressed in LtK- cells |
Bioorg Med Chem Lett 7: 297-302 (1997)
Article DOI: 10.1016/S0960-894X(97)00002-4
BindingDB Entry DOI: 10.7270/Q2B8584G |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50290980

(2-[4-Benzo[1,3]dioxol-5-yl-2-(4-methoxy-phenyl)-5-...)Show SMILES CCOC(=O)C(CC(C)C)NC(=O)OC1(OC(=O)C(=C1Cc1cc(OC)c(OC)c(OC)c1)c1ccc2OCOc2c1)c1ccc(OC)cc1 |c:18| Show InChI InChI=1S/C37H41NO12/c1-8-46-34(39)27(15-21(2)3)38-36(41)50-37(24-10-12-25(42-4)13-11-24)26(16-22-17-30(43-5)33(45-7)31(18-22)44-6)32(35(40)49-37)23-9-14-28-29(19-23)48-20-47-28/h9-14,17-19,21,27H,8,15-16,20H2,1-7H3,(H,38,41) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for human Endothelin A receptor expressed in LtK- cells |
Bioorg Med Chem Lett 7: 297-302 (1997)
Article DOI: 10.1016/S0960-894X(97)00002-4
BindingDB Entry DOI: 10.7270/Q2B8584G |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50290984
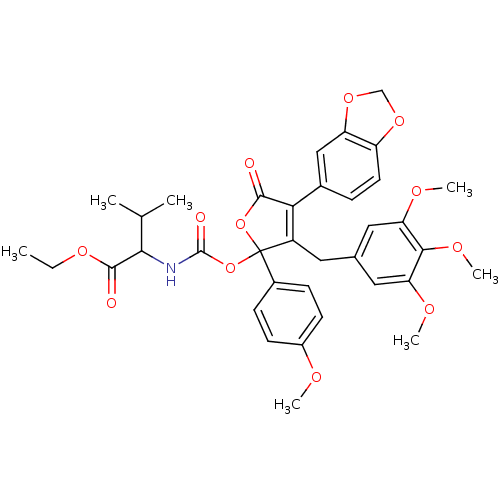
(2-[4-Benzo[1,3]dioxol-5-yl-2-(4-methoxy-phenyl)-5-...)Show SMILES CCOC(=O)C(NC(=O)OC1(OC(=O)C(=C1Cc1cc(OC)c(OC)c(OC)c1)c1ccc2OCOc2c1)c1ccc(OC)cc1)C(C)C |c:14| Show InChI InChI=1S/C36H39NO12/c1-8-45-34(39)31(20(2)3)37-35(40)49-36(23-10-12-24(41-4)13-11-23)25(15-21-16-28(42-5)32(44-7)29(17-21)43-6)30(33(38)48-36)22-9-14-26-27(18-22)47-19-46-26/h9-14,16-18,20,31H,8,15,19H2,1-7H3,(H,37,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for human Endothelin A receptor expressed in LtK- cells |
Bioorg Med Chem Lett 7: 297-302 (1997)
Article DOI: 10.1016/S0960-894X(97)00002-4
BindingDB Entry DOI: 10.7270/Q2B8584G |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50290982
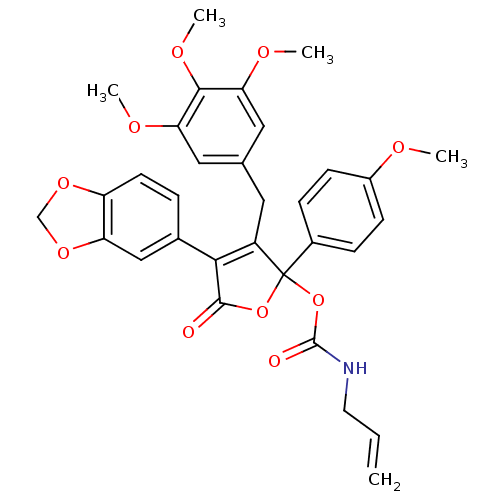
(Allyl-carbamic acid 4-benzo[1,3]dioxol-5-yl-2-(4-m...)Show SMILES COc1ccc(cc1)C1(OC(=O)NCC=C)OC(=O)C(=C1Cc1cc(OC)c(OC)c(OC)c1)c1ccc2OCOc2c1 |c:20| Show InChI InChI=1S/C32H31NO10/c1-6-13-33-31(35)43-32(21-8-10-22(36-2)11-9-21)23(14-19-15-26(37-3)29(39-5)27(16-19)38-4)28(30(34)42-32)20-7-12-24-25(17-20)41-18-40-24/h6-12,15-17H,1,13-14,18H2,2-5H3,(H,33,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for human Endothelin A receptor expressed in LtK- cells |
Bioorg Med Chem Lett 7: 297-302 (1997)
Article DOI: 10.1016/S0960-894X(97)00002-4
BindingDB Entry DOI: 10.7270/Q2B8584G |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50369268
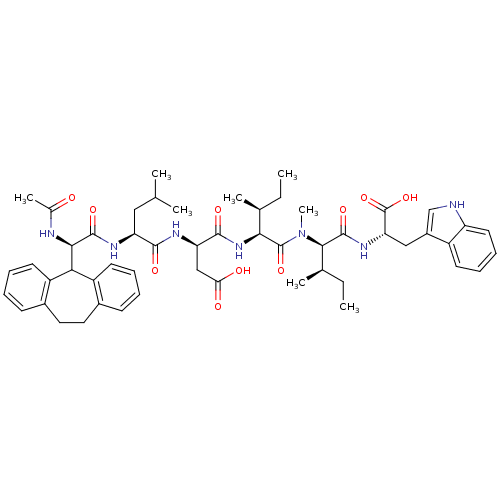
(CHEMBL1790519)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](NC(C)=O)C1c2ccccc2CCc2ccccc12)C(=O)N(C)[C@H]([C@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C53H69N7O10/c1-9-30(5)45(52(68)60(8)47(31(6)10-2)51(67)58-42(53(69)70)26-35-28-54-39-22-16-15-19-36(35)39)59-49(65)41(27-43(62)63)56-48(64)40(25-29(3)4)57-50(66)46(55-32(7)61)44-37-20-13-11-17-33(37)23-24-34-18-12-14-21-38(34)44/h11-22,28-31,40-42,44-47,54H,9-10,23-27H2,1-8H3,(H,55,61)(H,56,64)(H,57,66)(H,58,67)(H,59,65)(H,62,63)(H,69,70)/t30-,31+,40-,41+,42-,45-,46+,47+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of binding to Endothelin A receptor of rabbit renal vascular smooth muscle cells |
J Med Chem 40: 2228-40 (1997)
Article DOI: 10.1021/jm970161m
BindingDB Entry DOI: 10.7270/Q24T6K1W |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50290979

(((S)-1-Naphthalen-1-yl-ethyl)-carbamic acid 4-benz...)Show SMILES COc1ccc(cc1)C1(OC(=O)N[C@@H](C)c2cccc3ccccc23)OC(=O)C(=C1Cc1cc(OC)c(OC)c(OC)c1)c1ccc2OCOc2c1 |c:31| Show InChI InChI=1S/C41H37NO10/c1-24(30-12-8-10-26-9-6-7-11-31(26)30)42-40(44)52-41(28-14-16-29(45-2)17-15-28)32(19-25-20-35(46-3)38(48-5)36(21-25)47-4)37(39(43)51-41)27-13-18-33-34(22-27)50-23-49-33/h6-18,20-22,24H,19,23H2,1-5H3,(H,42,44)/t24-,41?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for human Endothelin A receptor expressed in LtK- cells |
Bioorg Med Chem Lett 7: 297-302 (1997)
Article DOI: 10.1016/S0960-894X(97)00002-4
BindingDB Entry DOI: 10.7270/Q2B8584G |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50290979

(((S)-1-Naphthalen-1-yl-ethyl)-carbamic acid 4-benz...)Show SMILES COc1ccc(cc1)C1(OC(=O)N[C@@H](C)c2cccc3ccccc23)OC(=O)C(=C1Cc1cc(OC)c(OC)c(OC)c1)c1ccc2OCOc2c1 |c:31| Show InChI InChI=1S/C41H37NO10/c1-24(30-12-8-10-26-9-6-7-11-31(26)30)42-40(44)52-41(28-14-16-29(45-2)17-15-28)32(19-25-20-35(46-3)38(48-5)36(21-25)47-4)37(39(43)51-41)27-13-18-33-34(22-27)50-23-49-33/h6-18,20-22,24H,19,23H2,1-5H3,(H,42,44)/t24-,41?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for human Endothelin A receptor expressed in LtK- cells |
Bioorg Med Chem Lett 7: 297-302 (1997)
Article DOI: 10.1016/S0960-894X(97)00002-4
BindingDB Entry DOI: 10.7270/Q2B8584G |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50032181
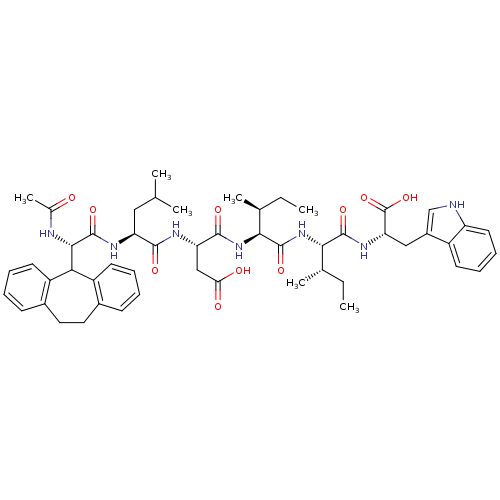
(2-{1-[1-(2-carboxy-1-{1-[10,11-dihydro-5H-dibenzo[...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(C)=O)C1c2ccccc2CCc2ccccc12)[C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C52H67N7O10/c1-8-29(5)44(49(65)57-41(52(68)69)25-34-27-53-38-21-15-14-18-35(34)38)59-50(66)45(30(6)9-2)58-48(64)40(26-42(61)62)55-47(63)39(24-28(3)4)56-51(67)46(54-31(7)60)43-36-19-12-10-16-32(36)22-23-33-17-11-13-20-37(33)43/h10-21,27-30,39-41,43-46,53H,8-9,22-26H2,1-7H3,(H,54,60)(H,55,63)(H,56,67)(H,57,65)(H,58,64)(H,59,66)(H,61,62)(H,68,69)/t29-,30-,39-,40-,41-,44-,45-,46-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of binding to Endothelin A receptor of rabbit renal vascular smooth muscle cells |
J Med Chem 40: 2228-40 (1997)
Article DOI: 10.1021/jm970161m
BindingDB Entry DOI: 10.7270/Q24T6K1W |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50057155
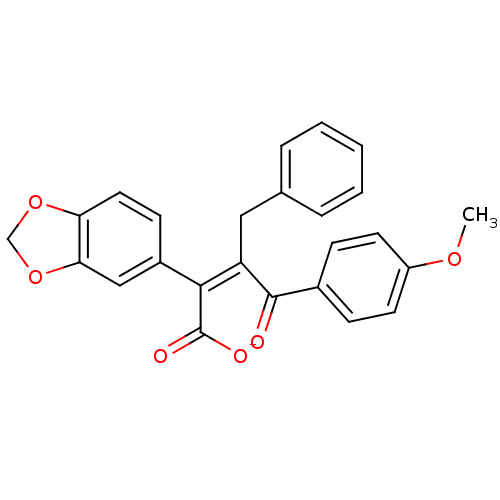
((Z)-2-Benzo[1,3]dioxol-5-yl-3-benzyl-4-(4-methoxy-...)Show SMILES COc1ccc(cc1)C(=O)C(\Cc1ccccc1)=C(/C([O-])=O)c1ccc2OCOc2c1 Show InChI InChI=1S/C25H20O6/c1-29-19-10-7-17(8-11-19)24(26)20(13-16-5-3-2-4-6-16)23(25(27)28)18-9-12-21-22(14-18)31-15-30-21/h2-12,14H,13,15H2,1H3,(H,27,28)/p-1/b23-20- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for human Endothelin A receptor expressed in LtK- cells |
Bioorg Med Chem Lett 7: 297-302 (1997)
Article DOI: 10.1016/S0960-894X(97)00002-4
BindingDB Entry DOI: 10.7270/Q2B8584G |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50290986

(CHEMBL320183 | Methyl-carbamic acid 4-benzo[1,3]di...)Show SMILES CNC(=O)OC1(OC(=O)C(=C1Cc1cc(OC)c(OC)c(OC)c1)c1ccc2OCOc2c1)c1ccc(OC)cc1 |c:9| Show InChI InChI=1S/C30H29NO10/c1-31-29(33)41-30(19-7-9-20(34-2)10-8-19)21(12-17-13-24(35-3)27(37-5)25(14-17)36-4)26(28(32)40-30)18-6-11-22-23(15-18)39-16-38-22/h6-11,13-15H,12,16H2,1-5H3,(H,31,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for human Endothelin A receptor expressed in LtK- cells |
Bioorg Med Chem Lett 7: 297-302 (1997)
Article DOI: 10.1016/S0960-894X(97)00002-4
BindingDB Entry DOI: 10.7270/Q2B8584G |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50032181

(2-{1-[1-(2-carboxy-1-{1-[10,11-dihydro-5H-dibenzo[...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(C)=O)C1c2ccccc2CCc2ccccc12)[C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C52H67N7O10/c1-8-29(5)44(49(65)57-41(52(68)69)25-34-27-53-38-21-15-14-18-35(34)38)59-50(66)45(30(6)9-2)58-48(64)40(26-42(61)62)55-47(63)39(24-28(3)4)56-51(67)46(54-31(7)60)43-36-19-12-10-16-32(36)22-23-33-17-11-13-20-37(33)43/h10-21,27-30,39-41,43-46,53H,8-9,22-26H2,1-7H3,(H,54,60)(H,55,63)(H,56,67)(H,57,65)(H,58,64)(H,59,66)(H,61,62)(H,68,69)/t29-,30-,39-,40-,41-,44-,45-,46-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration was evaluated by measuring the binding affinity at the Endothelin B receptor in rat cerebellar membranes |
J Med Chem 40: 2228-40 (1997)
Article DOI: 10.1021/jm970161m
BindingDB Entry DOI: 10.7270/Q24T6K1W |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50290985

(((S)-1-Phenyl-ethyl)-carbamic acid 4-benzo[1,3]dio...)Show SMILES COc1ccc(cc1)C1(OC(=O)N[C@@H](C)c2ccccc2)OC(=O)C(=C1Cc1ccccc1)c1ccc2OCOc2c1 |c:26| Show InChI InChI=1S/C34H29NO7/c1-22(24-11-7-4-8-12-24)35-33(37)42-34(26-14-16-27(38-2)17-15-26)28(19-23-9-5-3-6-10-23)31(32(36)41-34)25-13-18-29-30(20-25)40-21-39-29/h3-18,20,22H,19,21H2,1-2H3,(H,35,37)/t22-,34?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for human Endothelin A receptor expressed in LtK- cells |
Bioorg Med Chem Lett 7: 297-302 (1997)
Article DOI: 10.1016/S0960-894X(97)00002-4
BindingDB Entry DOI: 10.7270/Q2B8584G |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50369269

(CHEMBL1793932)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)Cc1c[nH]c2ccccc12)[C@@H](C)CC)C(=O)NCC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(C)=O)C(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C51H68N8O9/c1-8-30(5)44(59-50(67)45(31(6)9-2)58-47(64)37(52)25-35-27-53-38-23-17-16-22-36(35)38)49(66)54-28-41(61)39(26-42(62)63)56-48(65)40(24-29(3)4)57-51(68)46(55-32(7)60)43(33-18-12-10-13-19-33)34-20-14-11-15-21-34/h10-23,27,29-31,37,39-40,43-46,53H,8-9,24-26,28,52H2,1-7H3,(H,54,66)(H,55,60)(H,56,65)(H,57,68)(H,58,64)(H,59,67)(H,62,63)/t30-,31-,37-,39-,40-,44-,45-,46-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of binding to Endothelin B receptor of rat cerebellar membranes. |
J Med Chem 40: 2228-40 (1997)
Article DOI: 10.1021/jm970161m
BindingDB Entry DOI: 10.7270/Q24T6K1W |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50369268

(CHEMBL1790519)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](NC(C)=O)C1c2ccccc2CCc2ccccc12)C(=O)N(C)[C@H]([C@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C53H69N7O10/c1-9-30(5)45(52(68)60(8)47(31(6)10-2)51(67)58-42(53(69)70)26-35-28-54-39-22-16-15-19-36(35)39)59-49(65)41(27-43(62)63)56-48(64)40(25-29(3)4)57-50(66)46(55-32(7)61)44-37-20-13-11-17-33(37)23-24-34-18-12-14-21-38(34)44/h11-22,28-31,40-42,44-47,54H,9-10,23-27H2,1-8H3,(H,55,61)(H,56,64)(H,57,66)(H,58,67)(H,59,65)(H,62,63)(H,69,70)/t30-,31+,40-,41+,42-,45-,46+,47+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of binding to Endothelin B receptor of rat cerebellar membranes. |
J Med Chem 40: 2228-40 (1997)
Article DOI: 10.1021/jm970161m
BindingDB Entry DOI: 10.7270/Q24T6K1W |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50032172

((S)-3-[(S)-2-((S)-2-Acetylamino-3,3-diphenyl-propi...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(C)=O)C(c1ccccc1)c1ccccc1)[C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C50H65N7O10/c1-8-29(5)42(47(63)55-39(50(66)67)25-34-27-51-36-23-17-16-22-35(34)36)57-48(64)43(30(6)9-2)56-46(62)38(26-40(59)60)53-45(61)37(24-28(3)4)54-49(65)44(52-31(7)58)41(32-18-12-10-13-19-32)33-20-14-11-15-21-33/h10-23,27-30,37-39,41-44,51H,8-9,24-26H2,1-7H3,(H,52,58)(H,53,61)(H,54,65)(H,55,63)(H,56,62)(H,57,64)(H,59,60)(H,66,67)/t29-,30-,37-,38-,39-,42-,43-,44-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of binding to Endothelin A receptor of rabbit renal vascular smooth muscle cells |
J Med Chem 40: 2228-40 (1997)
Article DOI: 10.1021/jm970161m
BindingDB Entry DOI: 10.7270/Q24T6K1W |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50369267

(CHEMBL2369736)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(C)=O)C(c1ccccc1)c1ccccc1)C(=O)N(C)[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C51H67N7O10/c1-9-30(5)43(50(66)58(8)45(31(6)10-2)49(65)56-40(51(67)68)26-35-28-52-37-24-18-17-23-36(35)37)57-47(63)39(27-41(60)61)54-46(62)38(25-29(3)4)55-48(64)44(53-32(7)59)42(33-19-13-11-14-20-33)34-21-15-12-16-22-34/h11-24,28-31,38-40,42-45,52H,9-10,25-27H2,1-8H3,(H,53,59)(H,54,62)(H,55,64)(H,56,65)(H,57,63)(H,60,61)(H,67,68)/t30-,31-,38-,39-,40-,43-,44-,45-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of binding to Endothelin A receptor of rabbit renal vascular smooth muscle cells |
J Med Chem 40: 2228-40 (1997)
Article DOI: 10.1021/jm970161m
BindingDB Entry DOI: 10.7270/Q24T6K1W |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50369267

(CHEMBL2369736)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(C)=O)C(c1ccccc1)c1ccccc1)C(=O)N(C)[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C51H67N7O10/c1-9-30(5)43(50(66)58(8)45(31(6)10-2)49(65)56-40(51(67)68)26-35-28-52-37-24-18-17-23-36(35)37)57-47(63)39(27-41(60)61)54-46(62)38(25-29(3)4)55-48(64)44(53-32(7)59)42(33-19-13-11-14-20-33)34-21-15-12-16-22-34/h11-24,28-31,38-40,42-45,52H,9-10,25-27H2,1-8H3,(H,53,59)(H,54,62)(H,55,64)(H,56,65)(H,57,63)(H,60,61)(H,67,68)/t30-,31-,38-,39-,40-,43-,44-,45-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of binding to Endothelin B receptor of rat cerebellar membranes. |
J Med Chem 40: 2228-40 (1997)
Article DOI: 10.1021/jm970161m
BindingDB Entry DOI: 10.7270/Q24T6K1W |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50032172

((S)-3-[(S)-2-((S)-2-Acetylamino-3,3-diphenyl-propi...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(C)=O)C(c1ccccc1)c1ccccc1)[C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C50H65N7O10/c1-8-29(5)42(47(63)55-39(50(66)67)25-34-27-51-36-23-17-16-22-35(34)36)57-48(64)43(30(6)9-2)56-46(62)38(26-40(59)60)53-45(61)37(24-28(3)4)54-49(65)44(52-31(7)58)41(32-18-12-10-13-19-32)33-20-14-11-15-21-33/h10-23,27-30,37-39,41-44,51H,8-9,24-26H2,1-7H3,(H,52,58)(H,53,61)(H,54,65)(H,55,63)(H,56,62)(H,57,64)(H,59,60)(H,66,67)/t29-,30-,37-,38-,39-,42-,43-,44-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration was evaluated by measuring the binding affinity at the Endothelin B receptor in rat cerebellar membranes |
J Med Chem 40: 2228-40 (1997)
Article DOI: 10.1021/jm970161m
BindingDB Entry DOI: 10.7270/Q24T6K1W |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50369273

(CHEMBL1793926)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(C)=O)C(c1ccccc1)c1ccccc1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C51H67N7O10/c1-9-30(5)43(48(64)55-39(51(67)68)26-35-28-52-37-24-18-17-23-36(35)37)57-49(65)44(31(6)10-2)56-46(62)38(27-41(60)61)54-47(63)40(25-29(3)4)58(8)50(66)45(53-32(7)59)42(33-19-13-11-14-20-33)34-21-15-12-16-22-34/h11-24,28-31,38-40,42-45,52H,9-10,25-27H2,1-8H3,(H,53,59)(H,54,63)(H,55,64)(H,56,62)(H,57,65)(H,60,61)(H,67,68)/t30-,31-,38-,39-,40-,43-,44-,45-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of binding to Endothelin A receptor of rabbit renal vascular smooth muscle cells |
J Med Chem 40: 2228-40 (1997)
Article DOI: 10.1021/jm970161m
BindingDB Entry DOI: 10.7270/Q24T6K1W |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50369265

(CHEMBL1793928)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(C)=O)C(c1ccccc1)c1ccccc1)[C@H](C)CC)C(=O)CNC(=O)[C@H](N)Cc1c[nH]c2ccccc12 Show InChI InChI=1S/C51H68N8O9/c1-8-30(5)44(41(61)28-54-47(64)37(52)25-35-27-53-38-23-17-16-22-36(35)38)58-50(67)45(31(6)9-2)59-49(66)40(26-42(62)63)56-48(65)39(24-29(3)4)57-51(68)46(55-32(7)60)43(33-18-12-10-13-19-33)34-20-14-11-15-21-34/h10-23,27,29-31,37,39-40,43-46,53H,8-9,24-26,28,52H2,1-7H3,(H,54,64)(H,55,60)(H,56,65)(H,57,68)(H,58,67)(H,59,66)(H,62,63)/t30-,31+,37+,39-,40-,44-,45+,46-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >250 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of binding to Endothelin B receptor of rat cerebellar membranes. |
J Med Chem 40: 2228-40 (1997)
Article DOI: 10.1021/jm970161m
BindingDB Entry DOI: 10.7270/Q24T6K1W |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50369264
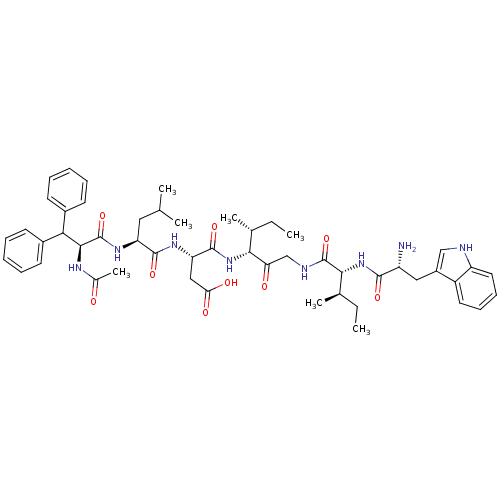
(CHEMBL1793929)Show SMILES CC[C@@H](C)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(C)=O)C(c1ccccc1)c1ccccc1)C(=O)CNC(=O)[C@H](NC(=O)[C@H](N)Cc1c[nH]c2ccccc12)[C@H](C)CC Show InChI InChI=1S/C51H68N8O9/c1-8-30(5)44(41(61)28-54-50(67)45(31(6)9-2)59-47(64)37(52)25-35-27-53-38-23-17-16-22-36(35)38)58-49(66)40(26-42(62)63)56-48(65)39(24-29(3)4)57-51(68)46(55-32(7)60)43(33-18-12-10-13-19-33)34-20-14-11-15-21-34/h10-23,27,29-31,37,39-40,43-46,53H,8-9,24-26,28,52H2,1-7H3,(H,54,67)(H,55,60)(H,56,65)(H,57,68)(H,58,66)(H,59,64)(H,62,63)/t30-,31-,37-,39+,40+,44-,45-,46+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >250 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of binding to Endothelin B receptor of rat cerebellar membranes. |
J Med Chem 40: 2228-40 (1997)
Article DOI: 10.1021/jm970161m
BindingDB Entry DOI: 10.7270/Q24T6K1W |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50369266

(CHEMBL1793925)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](N)Cc1c[nH]c2ccccc12)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(C)=O)C(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C51H68N8O9/c1-8-30(5)44(59-50(67)45(31(6)9-2)58-47(64)37(52)25-35-27-53-38-23-17-16-22-36(35)38)49(66)57-40(26-42(62)63)48(65)54-28-41(61)39(24-29(3)4)56-51(68)46(55-32(7)60)43(33-18-12-10-13-19-33)34-20-14-11-15-21-34/h10-23,27,29-31,37,39-40,43-46,53H,8-9,24-26,28,52H2,1-7H3,(H,54,65)(H,55,60)(H,56,68)(H,57,66)(H,58,64)(H,59,67)(H,62,63)/t30-,31-,37-,39-,40-,44-,45-,46-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >250 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of binding to Endothelin B receptor of rat cerebellar membranes. |
J Med Chem 40: 2228-40 (1997)
Article DOI: 10.1021/jm970161m
BindingDB Entry DOI: 10.7270/Q24T6K1W |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50369269

(CHEMBL1793932)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)Cc1c[nH]c2ccccc12)[C@@H](C)CC)C(=O)NCC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(C)=O)C(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C51H68N8O9/c1-8-30(5)44(59-50(67)45(31(6)9-2)58-47(64)37(52)25-35-27-53-38-23-17-16-22-36(35)38)49(66)54-28-41(61)39(26-42(62)63)56-48(65)40(24-29(3)4)57-51(68)46(55-32(7)60)43(33-18-12-10-13-19-33)34-20-14-11-15-21-34/h10-23,27,29-31,37,39-40,43-46,53H,8-9,24-26,28,52H2,1-7H3,(H,54,66)(H,55,60)(H,56,65)(H,57,68)(H,58,64)(H,59,67)(H,62,63)/t30-,31-,37-,39-,40-,44-,45-,46-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of binding to Endothelin A receptor of rabbit renal vascular smooth muscle cells |
J Med Chem 40: 2228-40 (1997)
Article DOI: 10.1021/jm970161m
BindingDB Entry DOI: 10.7270/Q24T6K1W |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50369264

(CHEMBL1793929)Show SMILES CC[C@@H](C)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(C)=O)C(c1ccccc1)c1ccccc1)C(=O)CNC(=O)[C@H](NC(=O)[C@H](N)Cc1c[nH]c2ccccc12)[C@H](C)CC Show InChI InChI=1S/C51H68N8O9/c1-8-30(5)44(41(61)28-54-50(67)45(31(6)9-2)59-47(64)37(52)25-35-27-53-38-23-17-16-22-36(35)38)58-49(66)40(26-42(62)63)56-48(65)39(24-29(3)4)57-51(68)46(55-32(7)60)43(33-18-12-10-13-19-33)34-20-14-11-15-21-34/h10-23,27,29-31,37,39-40,43-46,53H,8-9,24-26,28,52H2,1-7H3,(H,54,67)(H,55,60)(H,56,65)(H,57,68)(H,58,66)(H,59,64)(H,62,63)/t30-,31-,37-,39+,40+,44-,45-,46+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of binding to Endothelin A receptor of rabbit renal vascular smooth muscle cells |
J Med Chem 40: 2228-40 (1997)
Article DOI: 10.1021/jm970161m
BindingDB Entry DOI: 10.7270/Q24T6K1W |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50369266

(CHEMBL1793925)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](N)Cc1c[nH]c2ccccc12)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(C)=O)C(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C51H68N8O9/c1-8-30(5)44(59-50(67)45(31(6)9-2)58-47(64)37(52)25-35-27-53-38-23-17-16-22-36(35)38)49(66)57-40(26-42(62)63)48(65)54-28-41(61)39(24-29(3)4)56-51(68)46(55-32(7)60)43(33-18-12-10-13-19-33)34-20-14-11-15-21-34/h10-23,27,29-31,37,39-40,43-46,53H,8-9,24-26,28,52H2,1-7H3,(H,54,65)(H,55,60)(H,56,68)(H,57,66)(H,58,64)(H,59,67)(H,62,63)/t30-,31-,37-,39-,40-,44-,45-,46-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of binding to Endothelin A receptor of rabbit renal vascular smooth muscle cells |
J Med Chem 40: 2228-40 (1997)
Article DOI: 10.1021/jm970161m
BindingDB Entry DOI: 10.7270/Q24T6K1W |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50057176
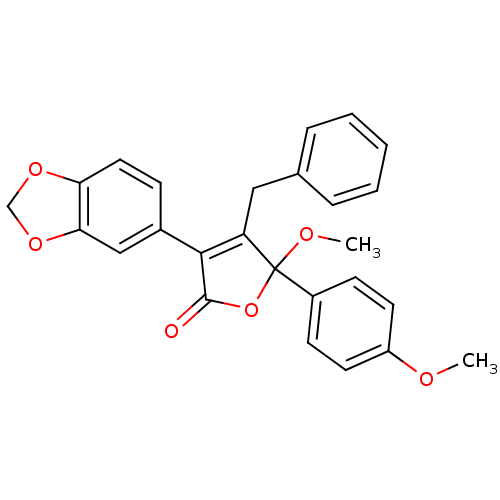
(3-Benzo[1,3]dioxol-5-yl-4-benzyl-5-methoxy-5-(4-me...)Show SMILES COc1ccc(cc1)C1(OC)OC(=O)C(=C1Cc1ccccc1)c1ccc2OCOc2c1 |c:15| Show InChI InChI=1S/C26H22O6/c1-28-20-11-9-19(10-12-20)26(29-2)21(14-17-6-4-3-5-7-17)24(25(27)32-26)18-8-13-22-23(15-18)31-16-30-22/h3-13,15H,14,16H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for human Endothelin A receptor expressed in LtK- cells |
Bioorg Med Chem Lett 7: 297-302 (1997)
Article DOI: 10.1016/S0960-894X(97)00002-4
BindingDB Entry DOI: 10.7270/Q2B8584G |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Homo sapiens (Human)) | BDBM50034267

(3-(benzo[d][1,3]dioxol-5-yl)-5-hydroxy-5-(4-methox...)Show SMILES COc1ccc(cc1)C1(O)OC(=O)C(=C1Cc1cc(OC)c(OC)c(OC)c1)c1ccc2OCOc2c1 |c:14| Show InChI InChI=1S/C28H26O9/c1-31-19-8-6-18(7-9-19)28(30)20(11-16-12-23(32-2)26(34-4)24(13-16)33-3)25(27(29)37-28)17-5-10-21-22(14-17)36-15-35-21/h5-10,12-14,30H,11,15H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for human endothelin B receptor expressed in CHO-K1 cells. |
Bioorg Med Chem Lett 7: 297-302 (1997)
Article DOI: 10.1016/S0960-894X(97)00002-4
BindingDB Entry DOI: 10.7270/Q2B8584G |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50060735
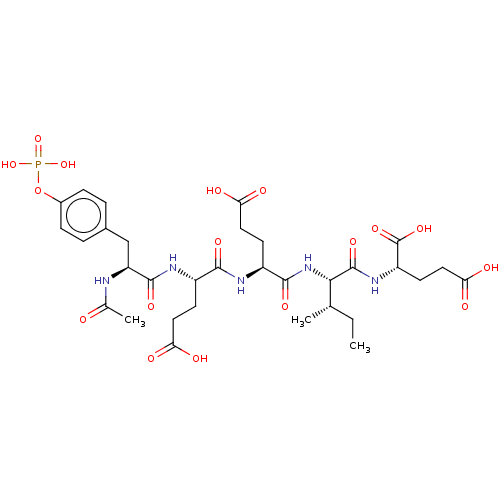
(2-[2-(2-{2-[(S)-2-Acetylamino-3-(4-phosphonooxy-ph...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccc(OP(O)(O)=O)cc1)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O Show InChI InChI=1S/C32H46N5O17P/c1-4-16(2)27(31(48)36-22(32(49)50)11-14-26(43)44)37-29(46)21(10-13-25(41)42)34-28(45)20(9-12-24(39)40)35-30(47)23(33-17(3)38)15-18-5-7-19(8-6-18)54-55(51,52)53/h5-8,16,20-23,27H,4,9-15H2,1-3H3,(H,33,38)(H,34,45)(H,35,47)(H,36,48)(H,37,46)(H,39,40)(H,41,42)(H,43,44)(H,49,50)(H2,51,52,53)/t16-,20-,21-,22-,23-,27-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Radioligand displacement assay for the binding of [125I]-Glu-Pro-Gln-pTyr-Glu-Glu-Ile-Pro-Ile-Tyr-Leu to SRC SH2 domain |
Bioorg Med Chem Lett 7: 1909-1914 (1997)
Article DOI: 10.1016/S0960-894X(97)00334-X
BindingDB Entry DOI: 10.7270/Q26110BP |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50369273

(CHEMBL1793926)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(C)=O)C(c1ccccc1)c1ccccc1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C51H67N7O10/c1-9-30(5)43(48(64)55-39(51(67)68)26-35-28-52-37-24-18-17-23-36(35)37)57-49(65)44(31(6)10-2)56-46(62)38(27-41(60)61)54-47(63)40(25-29(3)4)58(8)50(66)45(53-32(7)59)42(33-19-13-11-14-20-33)34-21-15-12-16-22-34/h11-24,28-31,38-40,42-45,52H,9-10,25-27H2,1-8H3,(H,53,59)(H,54,63)(H,55,64)(H,56,62)(H,57,65)(H,60,61)(H,67,68)/t30-,31-,38-,39-,40-,43-,44-,45-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of binding to Endothelin B receptor of rat cerebellar membranes. |
J Med Chem 40: 2228-40 (1997)
Article DOI: 10.1021/jm970161m
BindingDB Entry DOI: 10.7270/Q24T6K1W |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50369270
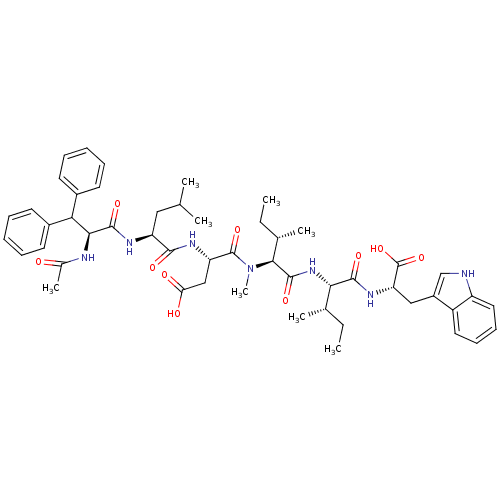
(CHEMBL1793933)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H]([C@@H](C)CC)N(C)C(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(C)=O)C(c1ccccc1)c1ccccc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C51H67N7O10/c1-9-30(5)43(47(63)56-40(51(67)68)26-35-28-52-37-24-18-17-23-36(35)37)57-49(65)45(31(6)10-2)58(8)50(66)39(27-41(60)61)55-46(62)38(25-29(3)4)54-48(64)44(53-32(7)59)42(33-19-13-11-14-20-33)34-21-15-12-16-22-34/h11-24,28-31,38-40,42-45,52H,9-10,25-27H2,1-8H3,(H,53,59)(H,54,64)(H,55,62)(H,56,63)(H,57,65)(H,60,61)(H,67,68)/t30-,31-,38-,39-,40-,43-,44-,45-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of binding to Endothelin A receptor of rabbit renal vascular smooth muscle cells |
J Med Chem 40: 2228-40 (1997)
Article DOI: 10.1021/jm970161m
BindingDB Entry DOI: 10.7270/Q24T6K1W |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50289743
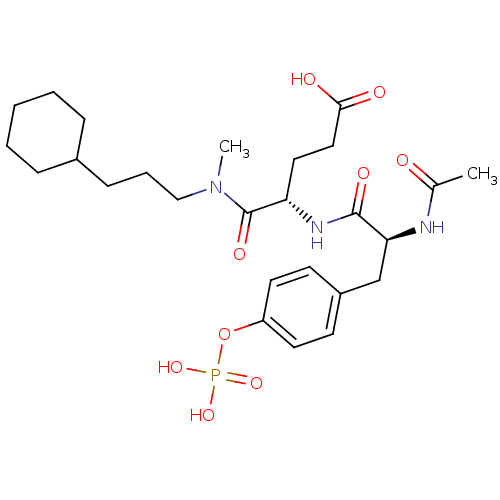
((S)-4-[(S)-2-Acetylamino-3-(4-phosphonooxy-phenyl)...)Show SMILES CN(CCCC1CCCCC1)C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccc(OP(O)(O)=O)cc1)NC(C)=O Show InChI InChI=1S/C26H40N3O9P/c1-18(30)27-23(17-20-10-12-21(13-11-20)38-39(35,36)37)25(33)28-22(14-15-24(31)32)26(34)29(2)16-6-9-19-7-4-3-5-8-19/h10-13,19,22-23H,3-9,14-17H2,1-2H3,(H,27,30)(H,28,33)(H,31,32)(H2,35,36,37)/t22-,23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [35S]-labeled SH2-GST Abl binding to the phospho-PDGF receptor intracellular domain |
Bioorg Med Chem Lett 7: 1909-1914 (1997)
Article DOI: 10.1016/S0960-894X(97)00334-X
BindingDB Entry DOI: 10.7270/Q26110BP |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50289749
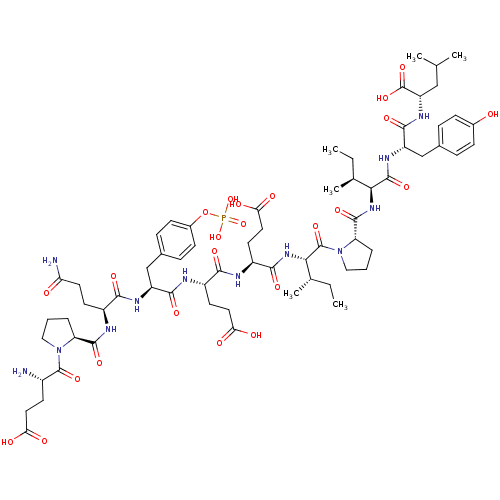
(CHEMBL414123 | Glu-Pro-Gln-pTyr-Glu-Glu-Ile-Pro-Il...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccc(OP(O)(O)=O)cc1)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CCC(O)=O)[C@@H](C)CC)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C66H97N12O24P/c1-7-35(5)54(63(94)73-46(32-37-13-17-39(79)18-14-37)60(91)74-47(66(97)98)31-34(3)4)75-62(93)49-12-10-30-78(49)65(96)55(36(6)8-2)76-58(89)44(24-28-53(85)86)69-56(87)43(23-27-52(83)84)70-59(90)45(33-38-15-19-40(20-16-38)102-103(99,100)101)72-57(88)42(22-25-50(68)80)71-61(92)48-11-9-29-77(48)64(95)41(67)21-26-51(81)82/h13-20,34-36,41-49,54-55,79H,7-12,21-33,67H2,1-6H3,(H2,68,80)(H,69,87)(H,70,90)(H,71,92)(H,72,88)(H,73,94)(H,74,91)(H,75,93)(H,76,89)(H,81,82)(H,83,84)(H,85,86)(H,97,98)(H2,99,100,101)/t35-,36-,41-,42-,43-,44-,45-,46-,47-,48-,49-,54-,55-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [35S]-labeled SH2-GST Abl binding to the phospho-PDGF receptor intracellular domain |
Bioorg Med Chem Lett 7: 1909-1914 (1997)
Article DOI: 10.1016/S0960-894X(97)00334-X
BindingDB Entry DOI: 10.7270/Q26110BP |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50289749

(CHEMBL414123 | Glu-Pro-Gln-pTyr-Glu-Glu-Ile-Pro-Il...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccc(OP(O)(O)=O)cc1)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CCC(O)=O)[C@@H](C)CC)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C66H97N12O24P/c1-7-35(5)54(63(94)73-46(32-37-13-17-39(79)18-14-37)60(91)74-47(66(97)98)31-34(3)4)75-62(93)49-12-10-30-78(49)65(96)55(36(6)8-2)76-58(89)44(24-28-53(85)86)69-56(87)43(23-27-52(83)84)70-59(90)45(33-38-15-19-40(20-16-38)102-103(99,100)101)72-57(88)42(22-25-50(68)80)71-61(92)48-11-9-29-77(48)64(95)41(67)21-26-51(81)82/h13-20,34-36,41-49,54-55,79H,7-12,21-33,67H2,1-6H3,(H2,68,80)(H,69,87)(H,70,90)(H,71,92)(H,72,88)(H,73,94)(H,74,91)(H,75,93)(H,76,89)(H,81,82)(H,83,84)(H,85,86)(H,97,98)(H2,99,100,101)/t35-,36-,41-,42-,43-,44-,45-,46-,47-,48-,49-,54-,55-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Radioligand displacement assay for the binding of [125I]-Glu-Pro-Gln-pTyr-Glu-Glu-Ile-Pro-Ile-Tyr-Leu to SRC SH2 domain |
Bioorg Med Chem Lett 7: 1909-1914 (1997)
Article DOI: 10.1016/S0960-894X(97)00334-X
BindingDB Entry DOI: 10.7270/Q26110BP |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50369265

(CHEMBL1793928)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(C)=O)C(c1ccccc1)c1ccccc1)[C@H](C)CC)C(=O)CNC(=O)[C@H](N)Cc1c[nH]c2ccccc12 Show InChI InChI=1S/C51H68N8O9/c1-8-30(5)44(41(61)28-54-47(64)37(52)25-35-27-53-38-23-17-16-22-36(35)38)58-50(67)45(31(6)9-2)59-49(66)40(26-42(62)63)56-48(65)39(24-29(3)4)57-51(68)46(55-32(7)60)43(33-18-12-10-13-19-33)34-20-14-11-15-21-34/h10-23,27,29-31,37,39-40,43-46,53H,8-9,24-26,28,52H2,1-7H3,(H,54,64)(H,55,60)(H,56,65)(H,57,68)(H,58,67)(H,59,66)(H,62,63)/t30-,31+,37+,39-,40-,44-,45+,46-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of binding to Endothelin A receptor of rabbit renal vascular smooth muscle cells |
J Med Chem 40: 2228-40 (1997)
Article DOI: 10.1021/jm970161m
BindingDB Entry DOI: 10.7270/Q24T6K1W |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50369270

(CHEMBL1793933)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H]([C@@H](C)CC)N(C)C(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(C)=O)C(c1ccccc1)c1ccccc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C51H67N7O10/c1-9-30(5)43(47(63)56-40(51(67)68)26-35-28-52-37-24-18-17-23-36(35)37)57-49(65)45(31(6)10-2)58(8)50(66)39(27-41(60)61)55-46(62)38(25-29(3)4)54-48(64)44(53-32(7)59)42(33-19-13-11-14-20-33)34-21-15-12-16-22-34/h11-24,28-31,38-40,42-45,52H,9-10,25-27H2,1-8H3,(H,53,59)(H,54,64)(H,55,62)(H,56,63)(H,57,65)(H,60,61)(H,67,68)/t30-,31-,38-,39-,40-,43-,44-,45-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of binding to Endothelin B receptor of rat cerebellar membranes. |
J Med Chem 40: 2228-40 (1997)
Article DOI: 10.1021/jm970161m
BindingDB Entry DOI: 10.7270/Q24T6K1W |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50289748

((S)-2-{(2S,3S)-2-[(S)-2-((S)-2-{(S)-2-Acetylamino-...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccc(cc1)C(F)(F)P(O)(O)=O)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O Show InChI InChI=1S/C33H46F2N5O16P/c1-4-16(2)27(31(51)39-22(32(52)53)11-14-26(46)47)40-29(49)21(10-13-25(44)45)37-28(48)20(9-12-24(42)43)38-30(50)23(36-17(3)41)15-18-5-7-19(8-6-18)33(34,35)57(54,55)56/h5-8,16,20-23,27H,4,9-15H2,1-3H3,(H,36,41)(H,37,48)(H,38,50)(H,39,51)(H,40,49)(H,42,43)(H,44,45)(H,46,47)(H,52,53)(H2,54,55,56)/t16-,20-,21-,22-,23-,27-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [35S]-labeled SH2-GST Abl binding to the phospho-PDGF receptor intracellular domain |
Bioorg Med Chem Lett 7: 1909-1914 (1997)
Article DOI: 10.1016/S0960-894X(97)00334-X
BindingDB Entry DOI: 10.7270/Q26110BP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50289748

((S)-2-{(2S,3S)-2-[(S)-2-((S)-2-{(S)-2-Acetylamino-...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccc(cc1)C(F)(F)P(O)(O)=O)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O Show InChI InChI=1S/C33H46F2N5O16P/c1-4-16(2)27(31(51)39-22(32(52)53)11-14-26(46)47)40-29(49)21(10-13-25(44)45)37-28(48)20(9-12-24(42)43)38-30(50)23(36-17(3)41)15-18-5-7-19(8-6-18)33(34,35)57(54,55)56/h5-8,16,20-23,27H,4,9-15H2,1-3H3,(H,36,41)(H,37,48)(H,38,50)(H,39,51)(H,40,49)(H,42,43)(H,44,45)(H,46,47)(H,52,53)(H2,54,55,56)/t16-,20-,21-,22-,23-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Radioligand displacement assay for the binding of [125I]-Glu-Pro-Gln-pTyr-Glu-Glu-Ile-Pro-Ile-Tyr-Leu to ABL SH2 domain |
Bioorg Med Chem Lett 7: 1909-1914 (1997)
Article DOI: 10.1016/S0960-894X(97)00334-X
BindingDB Entry DOI: 10.7270/Q26110BP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50289747

((S)-4-{(S)-2-Acetylamino-3-[4-(difluoro-phosphono-...)Show SMILES CC(=O)N[C@@H](Cc1ccc(cc1)C(F)(F)P(O)(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC1CCCCC1)C(N)=O Show InChI InChI=1S/C27H39F2N4O9P/c1-16(34)31-22(15-18-7-10-19(11-8-18)27(28,29)43(40,41)42)26(39)33-21(13-14-23(35)36)25(38)32-20(24(30)37)12-9-17-5-3-2-4-6-17/h7-8,10-11,17,20-22H,2-6,9,12-15H2,1H3,(H2,30,37)(H,31,34)(H,32,38)(H,33,39)(H,35,36)(H2,40,41,42)/t20-,21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [35S]-labeled SH2-GST Src binding to phospho-PDGF receptor intracellular domain |
Bioorg Med Chem Lett 7: 1909-1914 (1997)
Article DOI: 10.1016/S0960-894X(97)00334-X
BindingDB Entry DOI: 10.7270/Q26110BP |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50369263
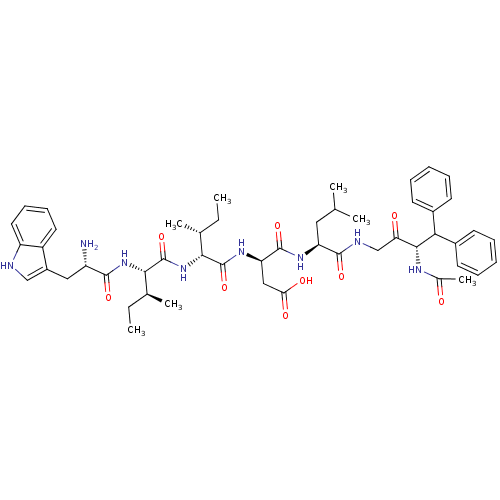
(CHEMBL1793924)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](N)Cc1c[nH]c2ccccc12)C(=O)N[C@H]([C@H](C)CC)C(=O)N[C@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)NCC(=O)[C@@H](NC(C)=O)C(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C51H68N8O9/c1-8-30(5)44(59-51(68)45(31(6)9-2)58-47(64)37(52)25-35-27-53-38-23-17-16-22-36(35)38)50(67)57-40(26-42(62)63)49(66)56-39(24-29(3)4)48(65)54-28-41(61)46(55-32(7)60)43(33-18-12-10-13-19-33)34-20-14-11-15-21-34/h10-23,27,29-31,37,39-40,43-46,53H,8-9,24-26,28,52H2,1-7H3,(H,54,65)(H,55,60)(H,56,66)(H,57,67)(H,58,64)(H,59,68)(H,62,63)/t30-,31+,37+,39+,40-,44-,45+,46-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of binding to Endothelin B receptor of rat cerebellar membranes. |
J Med Chem 40: 2228-40 (1997)
Article DOI: 10.1021/jm970161m
BindingDB Entry DOI: 10.7270/Q24T6K1W |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Homo sapiens (Human)) | BDBM50290984

(2-[4-Benzo[1,3]dioxol-5-yl-2-(4-methoxy-phenyl)-5-...)Show SMILES CCOC(=O)C(NC(=O)OC1(OC(=O)C(=C1Cc1cc(OC)c(OC)c(OC)c1)c1ccc2OCOc2c1)c1ccc(OC)cc1)C(C)C |c:14| Show InChI InChI=1S/C36H39NO12/c1-8-45-34(39)31(20(2)3)37-35(40)49-36(23-10-12-24(41-4)13-11-23)25(15-21-16-28(42-5)32(44-7)29(17-21)43-6)30(33(38)48-36)22-9-14-26-27(18-22)47-19-46-26/h9-14,16-18,20,31H,8,15,19H2,1-7H3,(H,37,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for human endothelin B receptor expressed in CHO-K1 cells. |
Bioorg Med Chem Lett 7: 297-302 (1997)
Article DOI: 10.1016/S0960-894X(97)00002-4
BindingDB Entry DOI: 10.7270/Q2B8584G |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50289747

((S)-4-{(S)-2-Acetylamino-3-[4-(difluoro-phosphono-...)Show SMILES CC(=O)N[C@@H](Cc1ccc(cc1)C(F)(F)P(O)(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC1CCCCC1)C(N)=O Show InChI InChI=1S/C27H39F2N4O9P/c1-16(34)31-22(15-18-7-10-19(11-8-18)27(28,29)43(40,41)42)26(39)33-21(13-14-23(35)36)25(38)32-20(24(30)37)12-9-17-5-3-2-4-6-17/h7-8,10-11,17,20-22H,2-6,9,12-15H2,1H3,(H2,30,37)(H,31,34)(H,32,38)(H,33,39)(H,35,36)(H2,40,41,42)/t20-,21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [35S]-labeled SH2-GST Abl binding to the phospho-PDGF receptor intracellular domain |
Bioorg Med Chem Lett 7: 1909-1914 (1997)
Article DOI: 10.1016/S0960-894X(97)00334-X
BindingDB Entry DOI: 10.7270/Q26110BP |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50060735

(2-[2-(2-{2-[(S)-2-Acetylamino-3-(4-phosphonooxy-ph...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccc(OP(O)(O)=O)cc1)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O Show InChI InChI=1S/C32H46N5O17P/c1-4-16(2)27(31(48)36-22(32(49)50)11-14-26(43)44)37-29(46)21(10-13-25(41)42)34-28(45)20(9-12-24(39)40)35-30(47)23(33-17(3)38)15-18-5-7-19(8-6-18)54-55(51,52)53/h5-8,16,20-23,27H,4,9-15H2,1-3H3,(H,33,38)(H,34,45)(H,35,47)(H,36,48)(H,37,46)(H,39,40)(H,41,42)(H,43,44)(H,49,50)(H2,51,52,53)/t16-,20-,21-,22-,23-,27-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [35S]-labeled SH2-GST Src binding to phospho-PDGF receptor intracellular domain |
Bioorg Med Chem Lett 7: 1909-1914 (1997)
Article DOI: 10.1016/S0960-894X(97)00334-X
BindingDB Entry DOI: 10.7270/Q26110BP |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50369271

(CHEMBL1793934)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(C)=O)C(c1ccccc1)c1ccccc1)[C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C51H67N7O10/c1-9-30(5)43(47(63)55-39(51(67)68)26-35-28-52-37-24-18-17-23-36(35)37)57-48(64)44(31(6)10-2)56-46(62)40(27-41(60)61)58(8)50(66)38(25-29(3)4)54-49(65)45(53-32(7)59)42(33-19-13-11-14-20-33)34-21-15-12-16-22-34/h11-24,28-31,38-40,42-45,52H,9-10,25-27H2,1-8H3,(H,53,59)(H,54,65)(H,55,63)(H,56,62)(H,57,64)(H,60,61)(H,67,68)/t30-,31-,38-,39-,40-,43-,44-,45-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of binding to Endothelin B receptor of rat cerebellar membranes. |
J Med Chem 40: 2228-40 (1997)
Article DOI: 10.1021/jm970161m
BindingDB Entry DOI: 10.7270/Q24T6K1W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50289746

(CHEMBL298813 | {[4-((S)-2-Acetylamino-2-{(S)-1-[(3...)Show SMILES CC[C@H](NC(=O)[C@H](Cc1ccc(cc1)C(F)(F)P(O)(O)=O)NC(C)=O)C(=O)N(C)CCCC1CCCCC1 Show InChI InChI=1S/C26H40F2N3O6P/c1-4-22(25(34)31(3)16-8-11-19-9-6-5-7-10-19)30-24(33)23(29-18(2)32)17-20-12-14-21(15-13-20)26(27,28)38(35,36)37/h12-15,19,22-23H,4-11,16-17H2,1-3H3,(H,29,32)(H,30,33)(H2,35,36,37)/t22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Radioligand displacement assay for the binding of [125I]-Glu-Pro-Gln-pTyr-Glu-Glu-Ile-Pro-Ile-Tyr-Leu to ABL SH2 domain |
Bioorg Med Chem Lett 7: 1909-1914 (1997)
Article DOI: 10.1016/S0960-894X(97)00334-X
BindingDB Entry DOI: 10.7270/Q26110BP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50289750
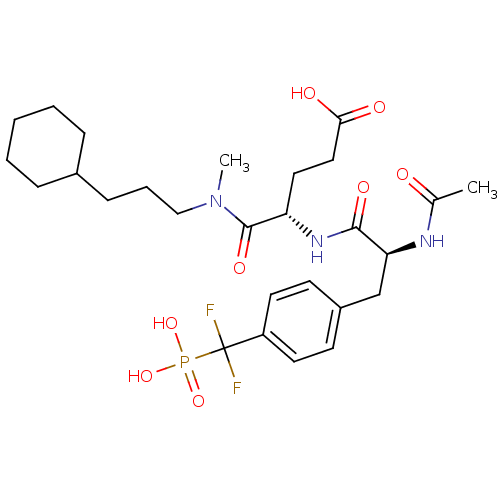
((S)-4-{(S)-2-Acetylamino-3-[4-(difluoro-phosphono-...)Show SMILES CN(CCCC1CCCCC1)C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccc(cc1)C(F)(F)P(O)(O)=O)NC(C)=O Show InChI InChI=1S/C27H40F2N3O8P/c1-18(33)30-23(17-20-10-12-21(13-11-20)27(28,29)41(38,39)40)25(36)31-22(14-15-24(34)35)26(37)32(2)16-6-9-19-7-4-3-5-8-19/h10-13,19,22-23H,3-9,14-17H2,1-2H3,(H,30,33)(H,31,36)(H,34,35)(H2,38,39,40)/t22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Radioligand displacement assay for the binding of [125I]-Glu-Pro-Gln-pTyr-Glu-Glu-Ile-Pro-Ile-Tyr-Leu to ABL SH2 domain |
Bioorg Med Chem Lett 7: 1909-1914 (1997)
Article DOI: 10.1016/S0960-894X(97)00334-X
BindingDB Entry DOI: 10.7270/Q26110BP |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Homo sapiens (Human)) | BDBM50290981

(CHEMBL319452 | PD-163140 | [4-Benzo[1,3]dioxol-5-y...)Show SMILES CCOC(=O)CNC(=O)OC1(OC(=O)C(=C1Cc1cc(OC)c(OC)c(OC)c1)c1ccc2OCOc2c1)c1ccc(OC)cc1 |c:14| Show InChI InChI=1S/C33H33NO12/c1-6-42-28(35)17-34-32(37)46-33(21-8-10-22(38-2)11-9-21)23(13-19-14-26(39-3)30(41-5)27(15-19)40-4)29(31(36)45-33)20-7-12-24-25(16-20)44-18-43-24/h7-12,14-16H,6,13,17-18H2,1-5H3,(H,34,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for human endothelin B receptor expressed in CHO-K1 cells. |
Bioorg Med Chem Lett 7: 297-302 (1997)
Article DOI: 10.1016/S0960-894X(97)00002-4
BindingDB Entry DOI: 10.7270/Q2B8584G |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50289752

(CHEMBL413629 | Glu-Pro-Gln-F2Pmp-Glu-Glu-Ile-Pro-I...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccc(cc1)C(F)(F)P(O)(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CCC(O)=O)[C@@H](C)CC)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C67H97F2N12O23P/c1-7-35(5)54(63(97)76-46(33-38-15-19-40(82)20-16-38)60(94)77-47(66(100)101)31-34(3)4)78-62(96)49-12-10-30-81(49)65(99)55(36(6)8-2)79-58(92)44(24-28-53(88)89)72-56(90)43(23-27-52(86)87)73-59(93)45(32-37-13-17-39(18-14-37)67(68,69)105(102,103)104)75-57(91)42(22-25-50(71)83)74-61(95)48-11-9-29-80(48)64(98)41(70)21-26-51(84)85/h13-20,34-36,41-49,54-55,82H,7-12,21-33,70H2,1-6H3,(H2,71,83)(H,72,90)(H,73,93)(H,74,95)(H,75,91)(H,76,97)(H,77,94)(H,78,96)(H,79,92)(H,84,85)(H,86,87)(H,88,89)(H,100,101)(H2,102,103,104)/t35-,36-,41-,42-,43-,44-,45-,46-,47-,48-,49-,54-,55-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Radioligand displacement assay for the binding of [125I]-Glu-Pro-Gln-pTyr-Glu-Glu-Ile-Pro-Ile-Tyr-Leu to SRC SH2 domain |
Bioorg Med Chem Lett 7: 1909-1914 (1997)
Article DOI: 10.1016/S0960-894X(97)00334-X
BindingDB Entry DOI: 10.7270/Q26110BP |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50289742

((S)-4-[(S)-2-Acetylamino-3-(4-phosphonooxy-phenyl)...)Show SMILES CC(=O)N[C@@H](Cc1ccc(OP(O)(O)=O)cc1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC1CCCCC1)C(N)=O Show InChI InChI=1S/C26H39N4O10P/c1-16(31)28-22(15-18-7-10-19(11-8-18)40-41(37,38)39)26(36)30-21(13-14-23(32)33)25(35)29-20(24(27)34)12-9-17-5-3-2-4-6-17/h7-8,10-11,17,20-22H,2-6,9,12-15H2,1H3,(H2,27,34)(H,28,31)(H,29,35)(H,30,36)(H,32,33)(H2,37,38,39)/t20-,21-,22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Radioligand displacement assay for the binding of [125I]-Glu-Pro-Gln-pTyr-Glu-Glu-Ile-Pro-Ile-Tyr-Leu to ABL SH2 domain |
Bioorg Med Chem Lett 7: 1909-1914 (1997)
Article DOI: 10.1016/S0960-894X(97)00334-X
BindingDB Entry DOI: 10.7270/Q26110BP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data