

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbapenem-hydrolyzing beta-lactamase KPC () | BDBM50569703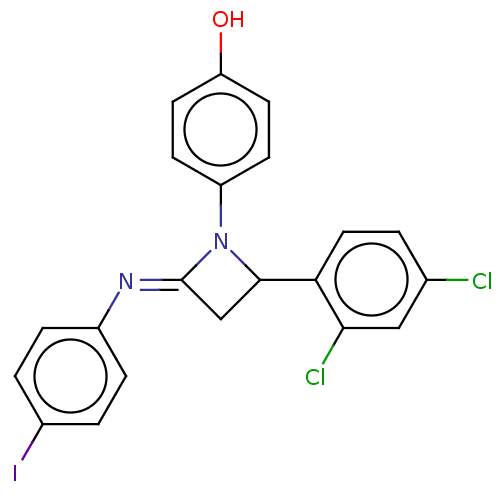 (CHEMBL4851703) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113418 BindingDB Entry DOI: 10.7270/Q26977C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbapenem-hydrolyzing beta-lactamase KPC () | BDBM50569685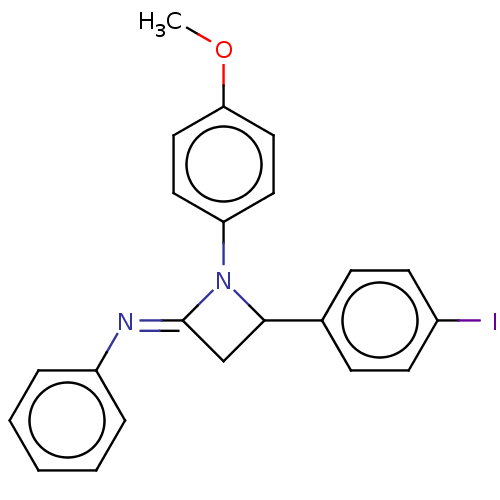 (CHEMBL4877631) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113418 BindingDB Entry DOI: 10.7270/Q26977C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbapenem-hydrolyzing beta-lactamase KPC () | BDBM50569681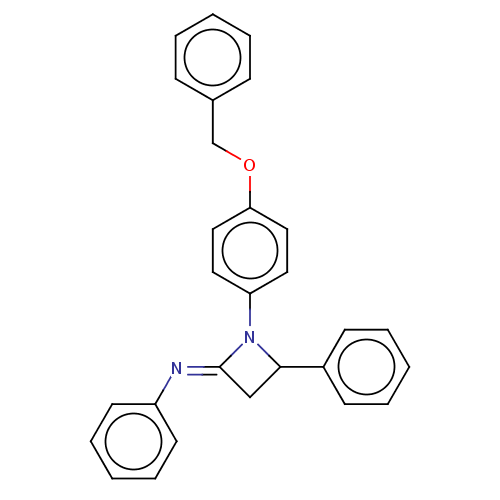 (CHEMBL4867398) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113418 BindingDB Entry DOI: 10.7270/Q26977C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbapenem-hydrolyzing beta-lactamase KPC () | BDBM50569680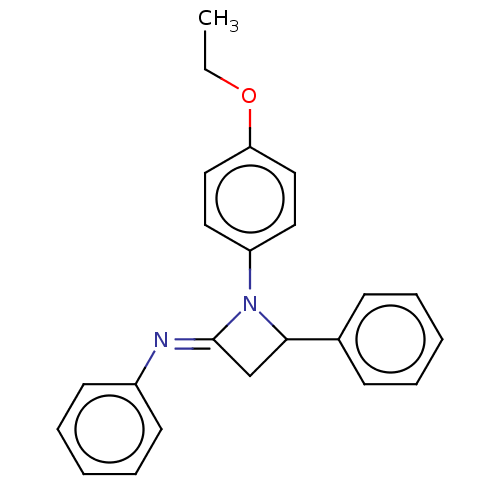 (CHEMBL4862416) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113418 BindingDB Entry DOI: 10.7270/Q26977C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbapenem-hydrolyzing beta-lactamase KPC () | BDBM50569686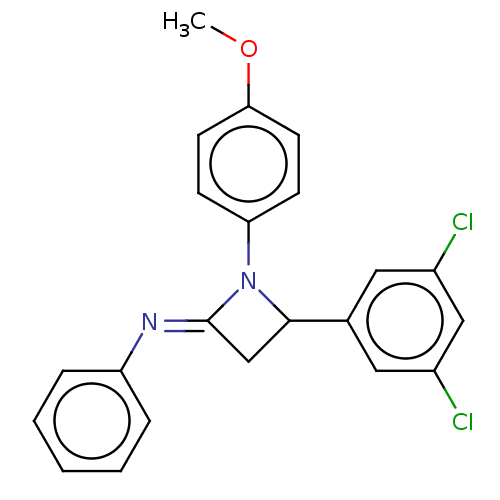 (CHEMBL4868540) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113418 BindingDB Entry DOI: 10.7270/Q26977C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbapenem-hydrolyzing beta-lactamase KPC () | BDBM50569689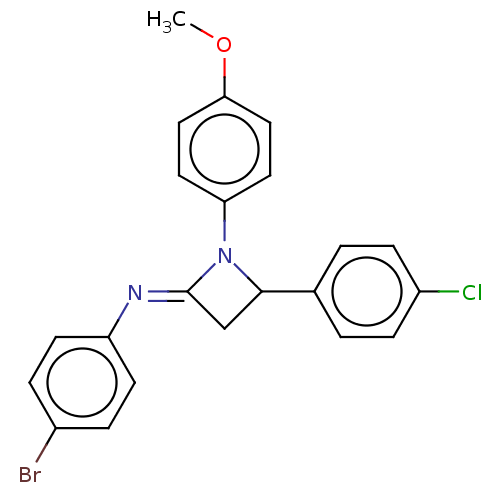 (CHEMBL4855728) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113418 BindingDB Entry DOI: 10.7270/Q26977C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbapenem-hydrolyzing beta-lactamase KPC () | BDBM50569696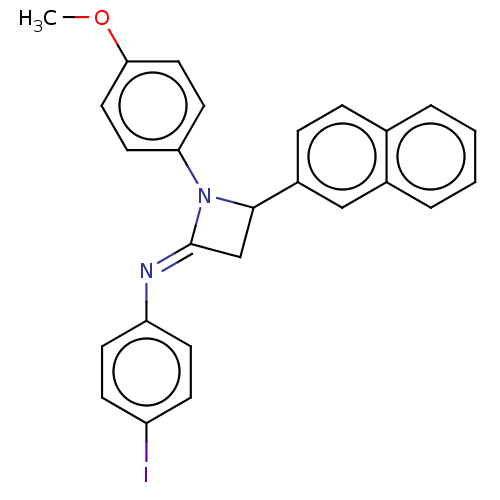 (CHEMBL4867248) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113418 BindingDB Entry DOI: 10.7270/Q26977C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbapenem-hydrolyzing beta-lactamase KPC () | BDBM50569690 (CHEMBL4876416) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113418 BindingDB Entry DOI: 10.7270/Q26977C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbapenem-hydrolyzing beta-lactamase KPC () | BDBM50569699 (CHEMBL4855195) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113418 BindingDB Entry DOI: 10.7270/Q26977C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbapenem-hydrolyzing beta-lactamase KPC () | BDBM50569698 (CHEMBL4855265) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113418 BindingDB Entry DOI: 10.7270/Q26977C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbapenem-hydrolyzing beta-lactamase KPC () | BDBM50569692 (CHEMBL4864504) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113418 BindingDB Entry DOI: 10.7270/Q26977C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbapenem-hydrolyzing beta-lactamase KPC () | BDBM50569688 (CHEMBL4848727) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113418 BindingDB Entry DOI: 10.7270/Q26977C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbapenem-hydrolyzing beta-lactamase KPC () | BDBM50569697 (CHEMBL4860974) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113418 BindingDB Entry DOI: 10.7270/Q26977C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbapenem-hydrolyzing beta-lactamase KPC () | BDBM50569687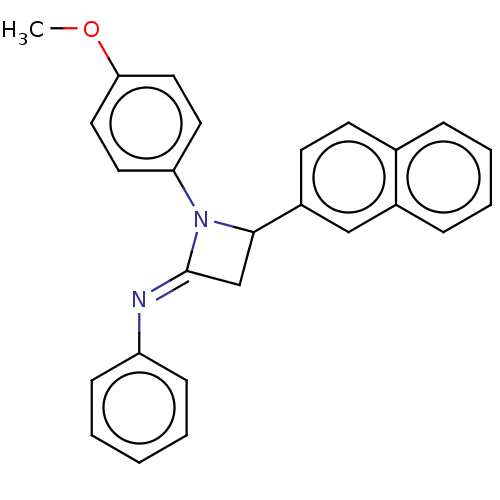 (CHEMBL4862171) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113418 BindingDB Entry DOI: 10.7270/Q26977C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbapenem-hydrolyzing beta-lactamase KPC () | BDBM50569691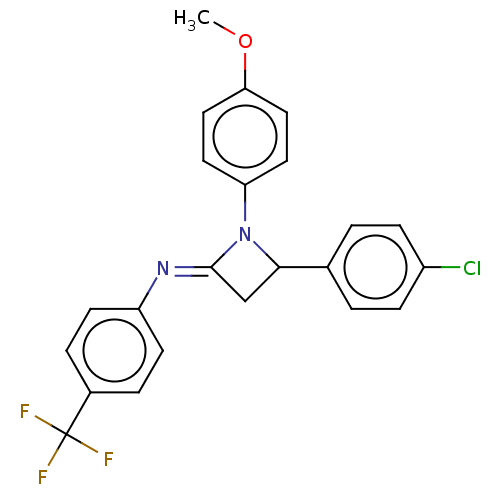 (CHEMBL4866009) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113418 BindingDB Entry DOI: 10.7270/Q26977C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbapenem-hydrolyzing beta-lactamase KPC () | BDBM50569684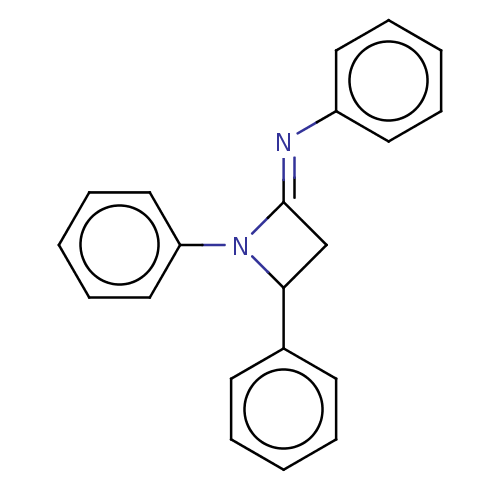 (CHEMBL4865343) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113418 BindingDB Entry DOI: 10.7270/Q26977C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbapenem-hydrolyzing beta-lactamase KPC () | BDBM50569682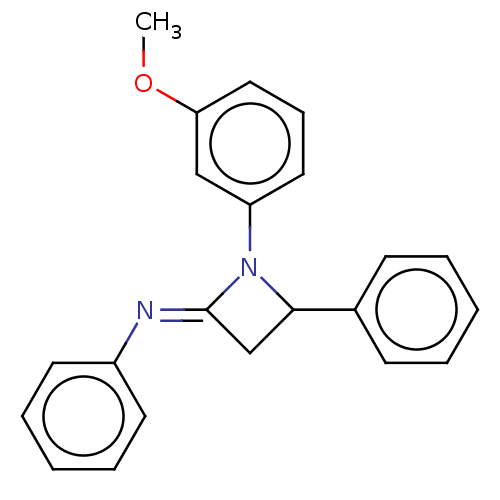 (CHEMBL4849131) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113418 BindingDB Entry DOI: 10.7270/Q26977C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbapenem-hydrolyzing beta-lactamase KPC () | BDBM50569679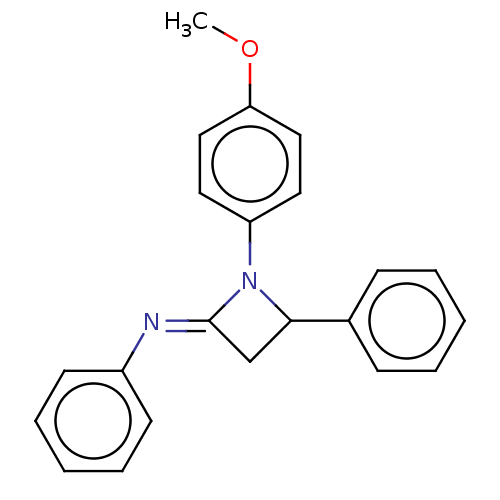 (CHEMBL4855927) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113418 BindingDB Entry DOI: 10.7270/Q26977C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbapenem-hydrolyzing beta-lactamase KPC () | BDBM50569683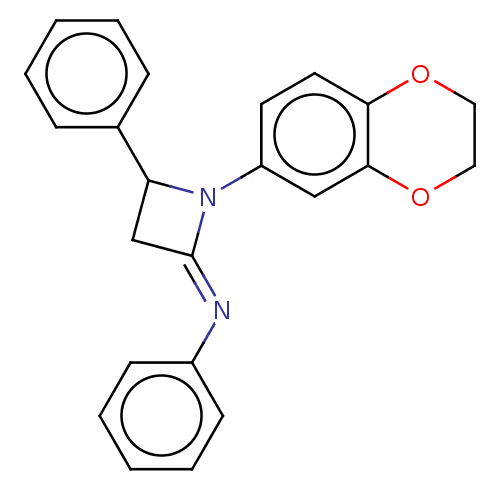 (CHEMBL4849406) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113418 BindingDB Entry DOI: 10.7270/Q26977C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbapenem-hydrolyzing beta-lactamase KPC () | BDBM50569702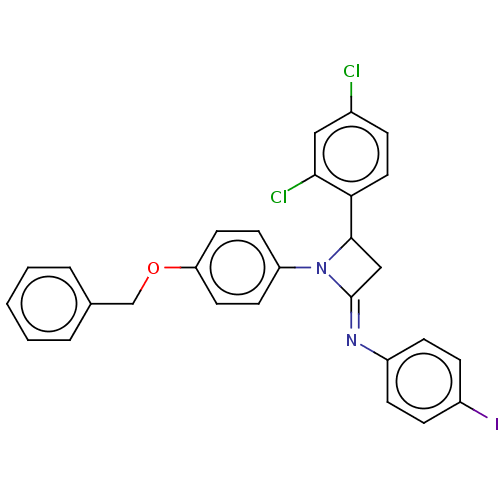 (CHEMBL4864325) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113418 BindingDB Entry DOI: 10.7270/Q26977C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbapenem-hydrolyzing beta-lactamase KPC () | BDBM50569704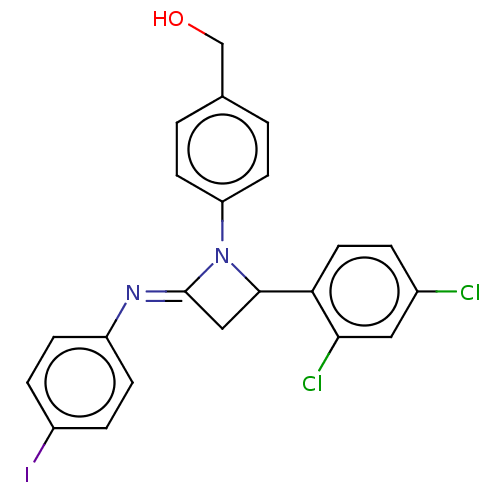 (CHEMBL4848912) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113418 BindingDB Entry DOI: 10.7270/Q26977C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbapenem-hydrolyzing beta-lactamase KPC () | BDBM50569694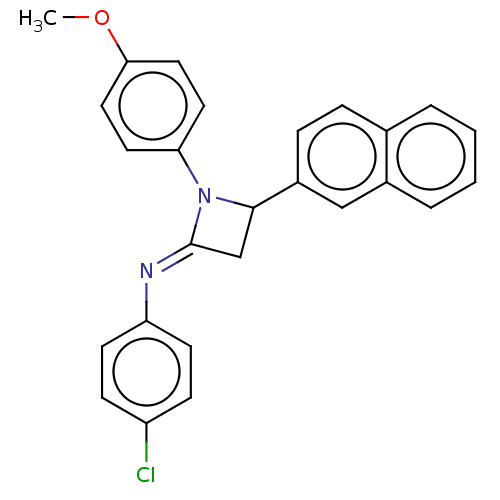 (CHEMBL4876790) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113418 BindingDB Entry DOI: 10.7270/Q26977C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbapenem-hydrolyzing beta-lactamase KPC () | BDBM50569705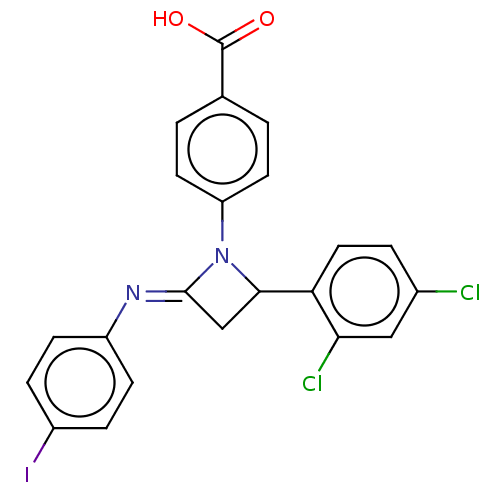 (CHEMBL4853896) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113418 BindingDB Entry DOI: 10.7270/Q26977C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbapenem-hydrolyzing beta-lactamase KPC () | BDBM50569695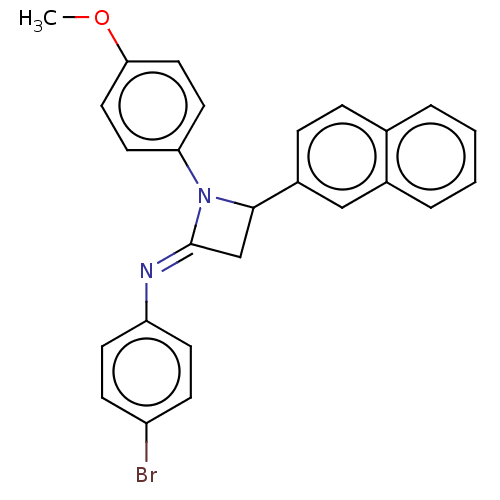 (CHEMBL4875502) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113418 BindingDB Entry DOI: 10.7270/Q26977C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbapenem-hydrolyzing beta-lactamase KPC () | BDBM50569701 (CHEMBL4872507) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113418 BindingDB Entry DOI: 10.7270/Q26977C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbapenem-hydrolyzing beta-lactamase KPC () | BDBM50569693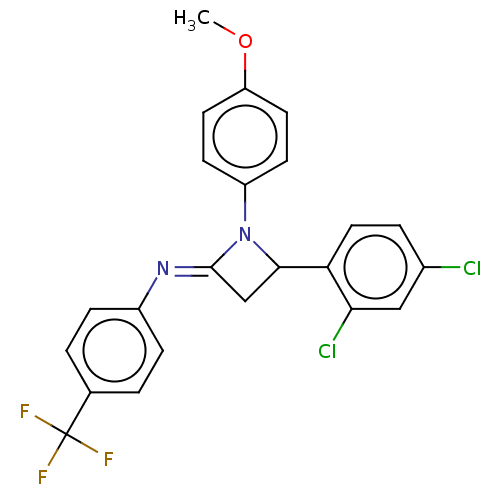 (CHEMBL4854293) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113418 BindingDB Entry DOI: 10.7270/Q26977C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbapenem-hydrolyzing beta-lactamase KPC () | BDBM50569700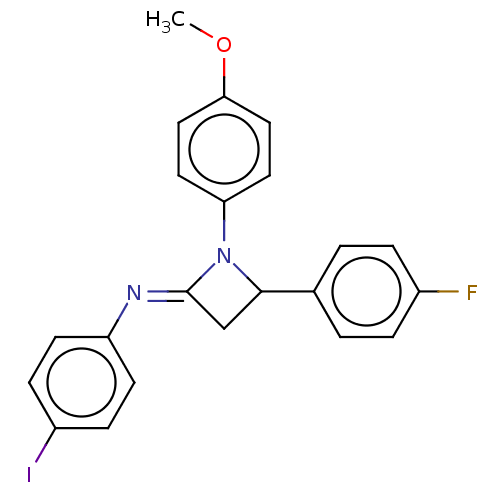 (CHEMBL4872541) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113418 BindingDB Entry DOI: 10.7270/Q26977C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50346357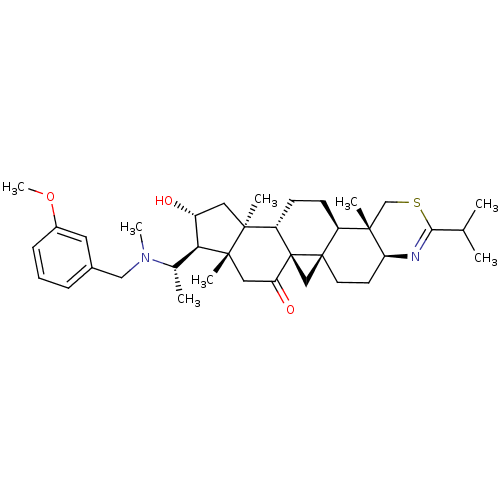 (20S-[(3-methoxybenzyl)(methyl)amino]-16alpha-hydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate by spectroscopic Ellmans method | Eur J Med Chem 46: 2193-205 (2011) Article DOI: 10.1016/j.ejmech.2011.02.073 BindingDB Entry DOI: 10.7270/Q2VM4CMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50346361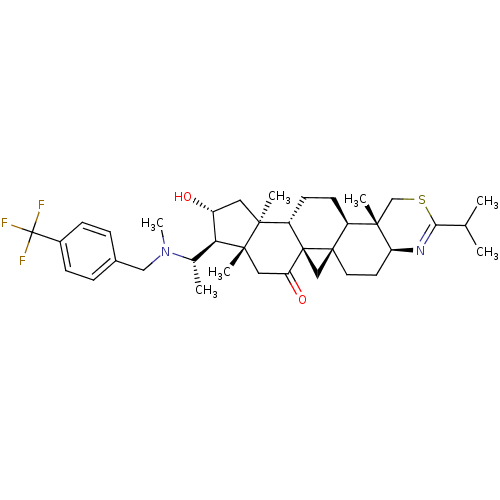 (20S-[p-(trifluoromethyl)benzylamino]-16alpha-hydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate by spectroscopic Ellmans method | Eur J Med Chem 46: 2193-205 (2011) Article DOI: 10.1016/j.ejmech.2011.02.073 BindingDB Entry DOI: 10.7270/Q2VM4CMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50346360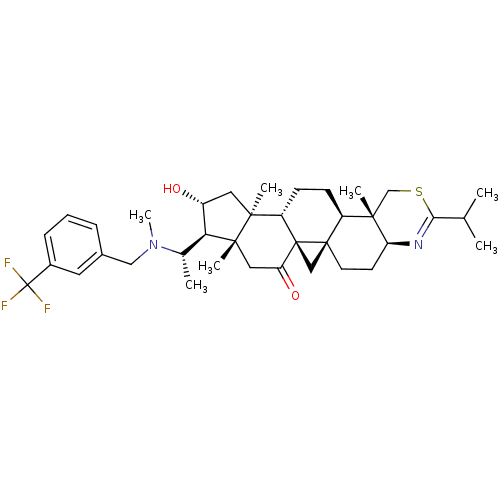 (20S-[m-(trifluoromethyl)benzylamino]-16alpha-hydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate by spectroscopic Ellmans method | Eur J Med Chem 46: 2193-205 (2011) Article DOI: 10.1016/j.ejmech.2011.02.073 BindingDB Entry DOI: 10.7270/Q2VM4CMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50346357 (20S-[(3-methoxybenzyl)(methyl)amino]-16alpha-hydro...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate by spectroscopic Ellmans method | Eur J Med Chem 46: 2193-205 (2011) Article DOI: 10.1016/j.ejmech.2011.02.073 BindingDB Entry DOI: 10.7270/Q2VM4CMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50346356 (20S-[(2-methoxybenzyl)(methyl)amino]-16alpha-hydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate by spectroscopic Ellmans method | Eur J Med Chem 46: 2193-205 (2011) Article DOI: 10.1016/j.ejmech.2011.02.073 BindingDB Entry DOI: 10.7270/Q2VM4CMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50346353 (20S-[benzyl(methyl)amino]-16alpha-hydroxy-4beta,14...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate by spectroscopic Ellmans method | Eur J Med Chem 46: 2193-205 (2011) Article DOI: 10.1016/j.ejmech.2011.02.073 BindingDB Entry DOI: 10.7270/Q2VM4CMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50346361 (20S-[p-(trifluoromethyl)benzylamino]-16alpha-hydro...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate by spectroscopic Ellmans method | Eur J Med Chem 46: 2193-205 (2011) Article DOI: 10.1016/j.ejmech.2011.02.073 BindingDB Entry DOI: 10.7270/Q2VM4CMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50346358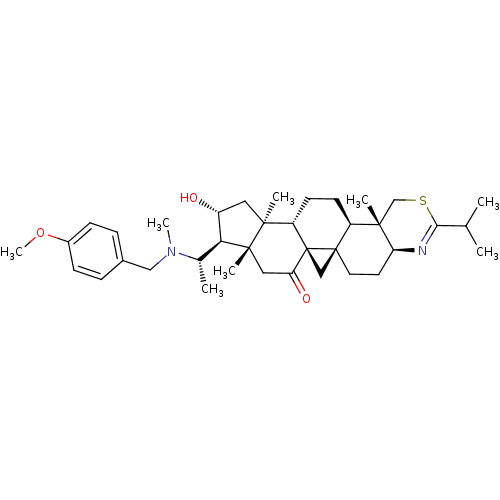 (20S-[(4-methoxybenzyl)(methyl)amino]-16alpha-hydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate by spectroscopic Ellmans method | Eur J Med Chem 46: 2193-205 (2011) Article DOI: 10.1016/j.ejmech.2011.02.073 BindingDB Entry DOI: 10.7270/Q2VM4CMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50346360 (20S-[m-(trifluoromethyl)benzylamino]-16alpha-hydro...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate by spectroscopic Ellmans method | Eur J Med Chem 46: 2193-205 (2011) Article DOI: 10.1016/j.ejmech.2011.02.073 BindingDB Entry DOI: 10.7270/Q2VM4CMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50346359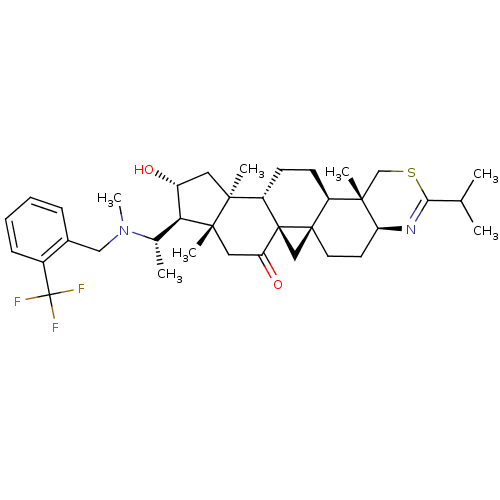 (20S-[o-(trifluoromethyl)benzylamino]-16alpha-hydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate by spectroscopic Ellmans method | Eur J Med Chem 46: 2193-205 (2011) Article DOI: 10.1016/j.ejmech.2011.02.073 BindingDB Entry DOI: 10.7270/Q2VM4CMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50346356 (20S-[(2-methoxybenzyl)(methyl)amino]-16alpha-hydro...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate by spectroscopic Ellmans method | Eur J Med Chem 46: 2193-205 (2011) Article DOI: 10.1016/j.ejmech.2011.02.073 BindingDB Entry DOI: 10.7270/Q2VM4CMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50346353 (20S-[benzyl(methyl)amino]-16alpha-hydroxy-4beta,14...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate by spectroscopic Ellmans method | Eur J Med Chem 46: 2193-205 (2011) Article DOI: 10.1016/j.ejmech.2011.02.073 BindingDB Entry DOI: 10.7270/Q2VM4CMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50346363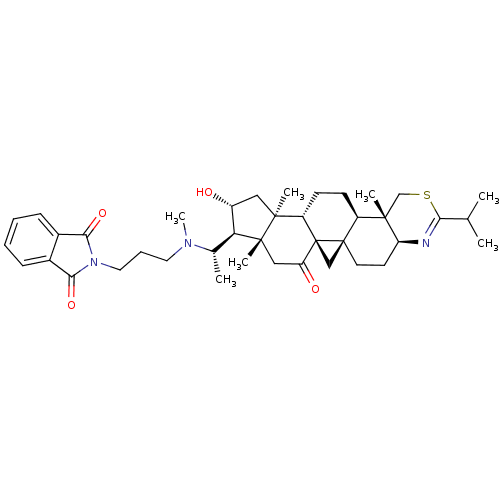 (2-{3-[[16alpha-hydroxy-4beta,14alpha-dimethyl-11-o...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate by spectroscopic Ellmans method | Eur J Med Chem 46: 2193-205 (2011) Article DOI: 10.1016/j.ejmech.2011.02.073 BindingDB Entry DOI: 10.7270/Q2VM4CMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50346363 (2-{3-[[16alpha-hydroxy-4beta,14alpha-dimethyl-11-o...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate by spectroscopic Ellmans method | Eur J Med Chem 46: 2193-205 (2011) Article DOI: 10.1016/j.ejmech.2011.02.073 BindingDB Entry DOI: 10.7270/Q2VM4CMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50346354 (20S-[methyl(2-phenylethyl)amino]-16alpha-hydroxy-4...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate by spectroscopic Ellmans method | Eur J Med Chem 46: 2193-205 (2011) Article DOI: 10.1016/j.ejmech.2011.02.073 BindingDB Entry DOI: 10.7270/Q2VM4CMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50346370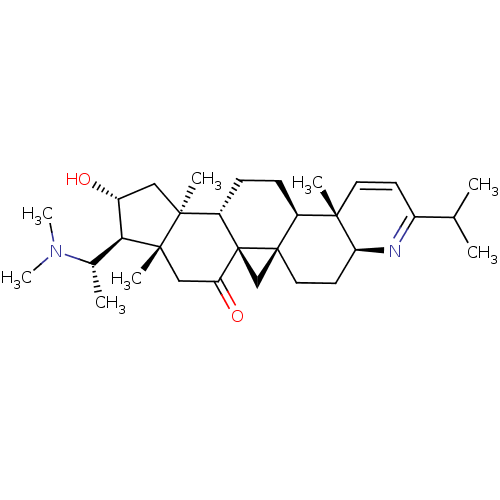 (20S-(dimethylamino)-16a-hydroxy-4beta,14alpha-dime...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate by spectroscopic Ellmans method | Eur J Med Chem 46: 2193-205 (2011) Article DOI: 10.1016/j.ejmech.2011.02.073 BindingDB Entry DOI: 10.7270/Q2VM4CMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50346358 (20S-[(4-methoxybenzyl)(methyl)amino]-16alpha-hydro...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate by spectroscopic Ellmans method | Eur J Med Chem 46: 2193-205 (2011) Article DOI: 10.1016/j.ejmech.2011.02.073 BindingDB Entry DOI: 10.7270/Q2VM4CMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50346354 (20S-[methyl(2-phenylethyl)amino]-16alpha-hydroxy-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate by spectroscopic Ellmans method | Eur J Med Chem 46: 2193-205 (2011) Article DOI: 10.1016/j.ejmech.2011.02.073 BindingDB Entry DOI: 10.7270/Q2VM4CMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50346359 (20S-[o-(trifluoromethyl)benzylamino]-16alpha-hydro...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate by spectroscopic Ellmans method | Eur J Med Chem 46: 2193-205 (2011) Article DOI: 10.1016/j.ejmech.2011.02.073 BindingDB Entry DOI: 10.7270/Q2VM4CMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50346362 (2-{2-[[16alpha-hydroxy-4beta,14alpha-dimethyl-11-o...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate by spectroscopic Ellmans method | Eur J Med Chem 46: 2193-205 (2011) Article DOI: 10.1016/j.ejmech.2011.02.073 BindingDB Entry DOI: 10.7270/Q2VM4CMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50346370 (20S-(dimethylamino)-16a-hydroxy-4beta,14alpha-dime...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate by spectroscopic Ellmans method | Eur J Med Chem 46: 2193-205 (2011) Article DOI: 10.1016/j.ejmech.2011.02.073 BindingDB Entry DOI: 10.7270/Q2VM4CMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50346351 (CHEMBL1783523 | N-{16alpha-hydroxy-4beta,14alpha-d...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate by spectroscopic Ellmans method | Eur J Med Chem 46: 2193-205 (2011) Article DOI: 10.1016/j.ejmech.2011.02.073 BindingDB Entry DOI: 10.7270/Q2VM4CMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50346372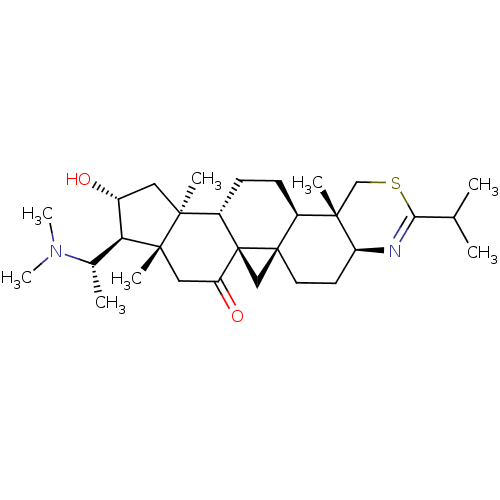 (20S-(dimethylamino)-16alpha-hydroxy-4beta,14alpha-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate by spectroscopic Ellmans method | Eur J Med Chem 46: 2193-205 (2011) Article DOI: 10.1016/j.ejmech.2011.02.073 BindingDB Entry DOI: 10.7270/Q2VM4CMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 232 total ) | Next | Last >> |