

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Substance-K receptor (Homo sapiens (Human)) | BDBM50472916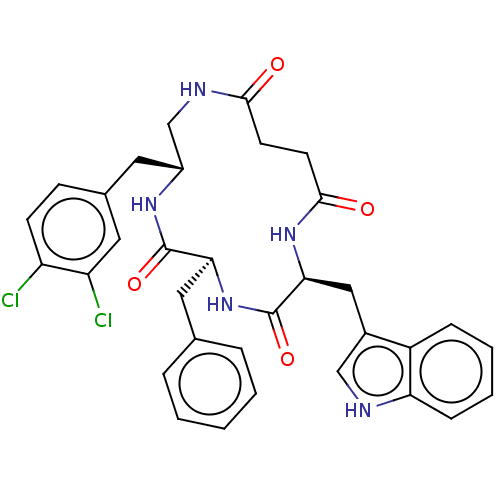 (CHEMBL223221 | MEN-11690) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Binding affinity towards Tachykinin receptor 2 | J Med Chem 43: 4041-4 (2000) Article DOI: 10.1021/jm0010217 BindingDB Entry DOI: 10.7270/Q2Z322CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50366377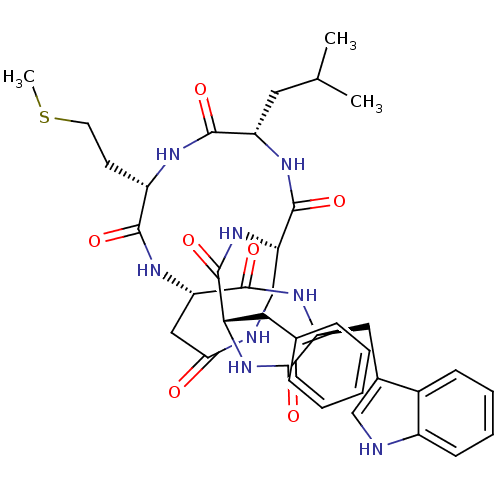 (CHEMBL334721 | MEN-10627) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Binding affinity towards Tachykinin receptor 2 | J Med Chem 43: 4041-4 (2000) Article DOI: 10.1021/jm0010217 BindingDB Entry DOI: 10.7270/Q2Z322CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50472917 (CHEMBL323884 | MEN-11749) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Binding affinity towards Tachykinin receptor 2 | J Med Chem 43: 4041-4 (2000) Article DOI: 10.1021/jm0010217 BindingDB Entry DOI: 10.7270/Q2Z322CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50404020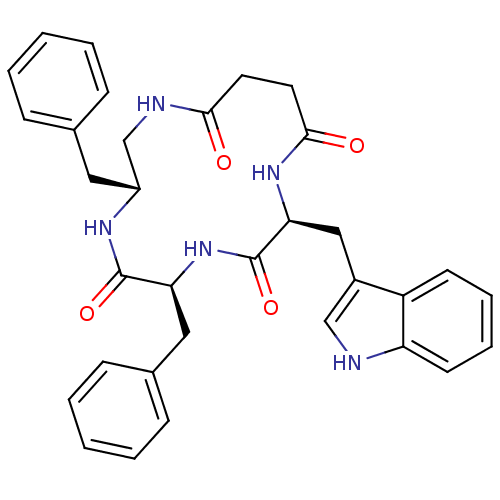 (CHEMBL435324 | MEN-11558) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Binding affinity towards Tachykinin receptor 2 | J Med Chem 43: 4041-4 (2000) Article DOI: 10.1021/jm0010217 BindingDB Entry DOI: 10.7270/Q2Z322CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50472911 (CHEMBL407782 | MEN-11420) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Binding affinity towards Tachykinin receptor 2 | J Med Chem 43: 4041-4 (2000) Article DOI: 10.1021/jm0010217 BindingDB Entry DOI: 10.7270/Q2Z322CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50472912 (CHEMBL116564 | MEN-11712) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Binding affinity towards Tachykinin receptor 2 | J Med Chem 43: 4041-4 (2000) Article DOI: 10.1021/jm0010217 BindingDB Entry DOI: 10.7270/Q2Z322CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50472910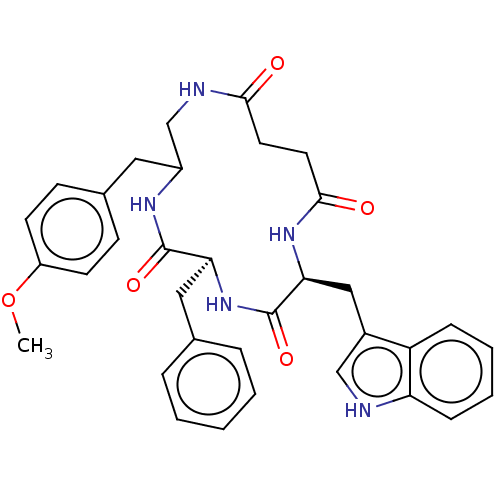 (CHEMBL263194) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Binding affinity towards Tachykinin receptor 2 | J Med Chem 43: 4041-4 (2000) Article DOI: 10.1021/jm0010217 BindingDB Entry DOI: 10.7270/Q2Z322CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50472908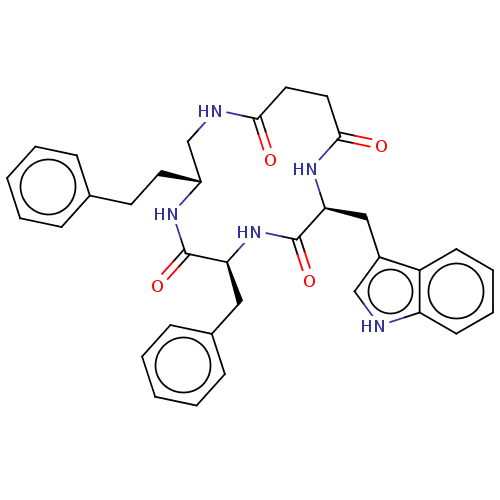 (CHEMBL336053) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Binding affinity towards Tachykinin receptor 2 | J Med Chem 43: 4041-4 (2000) Article DOI: 10.1021/jm0010217 BindingDB Entry DOI: 10.7270/Q2Z322CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50472913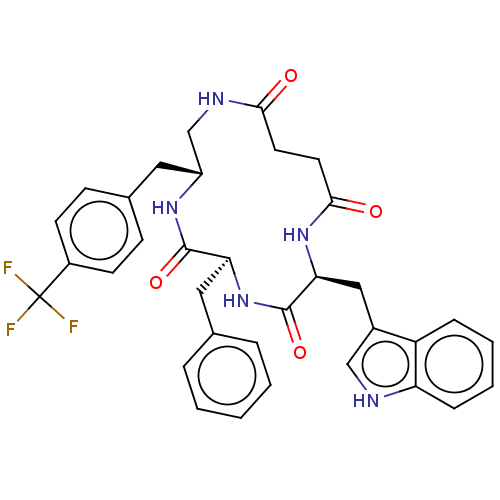 (CHEMBL2112675) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Binding affinity towards Tachykinin receptor 2 | J Med Chem 43: 4041-4 (2000) Article DOI: 10.1021/jm0010217 BindingDB Entry DOI: 10.7270/Q2Z322CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50472914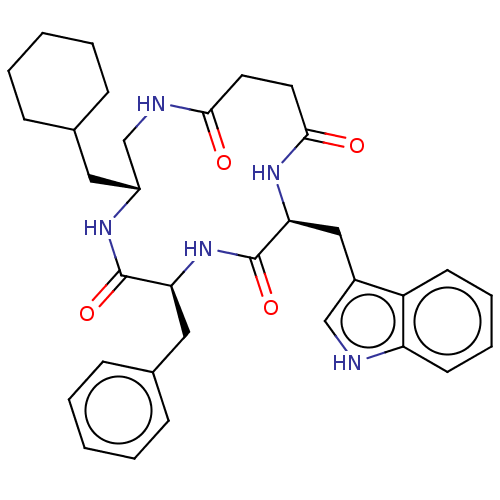 (CHEMBL114442 | MEN-11667) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Binding affinity towards Tachykinin receptor 2 | J Med Chem 43: 4041-4 (2000) Article DOI: 10.1021/jm0010217 BindingDB Entry DOI: 10.7270/Q2Z322CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50472919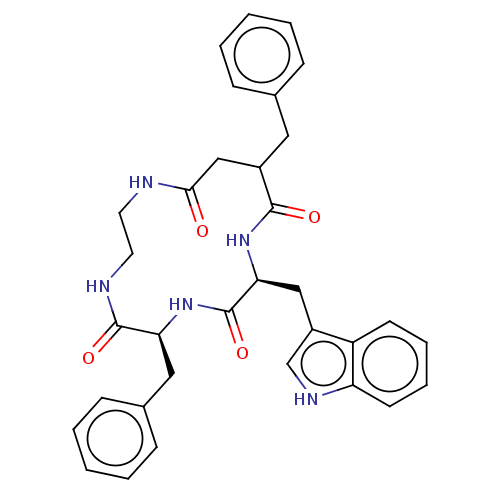 (CHEMBL337628) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Binding affinity towards Tachykinin receptor 2 | J Med Chem 43: 4041-4 (2000) Article DOI: 10.1021/jm0010217 BindingDB Entry DOI: 10.7270/Q2Z322CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50472915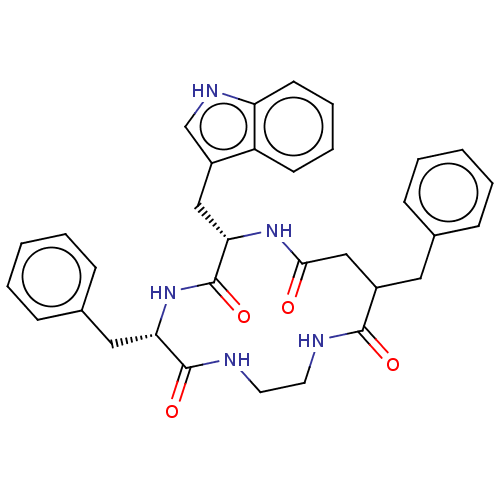 (CHEMBL114036 | MEN-11540) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Binding affinity towards Tachykinin receptor 2 | J Med Chem 43: 4041-4 (2000) Article DOI: 10.1021/jm0010217 BindingDB Entry DOI: 10.7270/Q2Z322CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50472918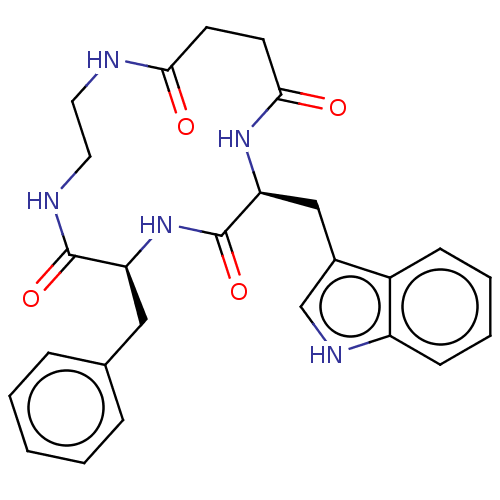 (CHEMBL324804 | MEN-11970) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Binding affinity towards Tachykinin receptor 2 | J Med Chem 43: 4041-4 (2000) Article DOI: 10.1021/jm0010217 BindingDB Entry DOI: 10.7270/Q2Z322CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50472906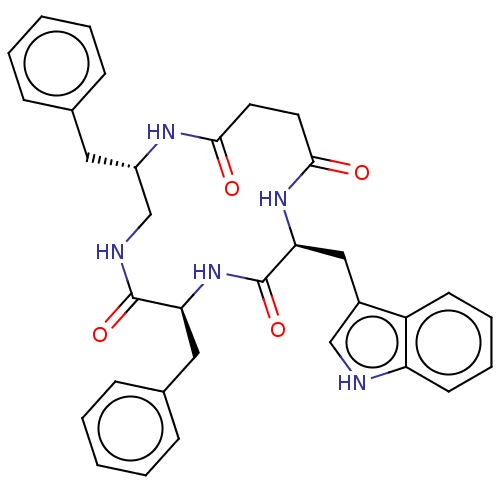 (CHEMBL2111858) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Binding affinity towards Tachykinin receptor 2 | J Med Chem 43: 4041-4 (2000) Article DOI: 10.1021/jm0010217 BindingDB Entry DOI: 10.7270/Q2Z322CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50472907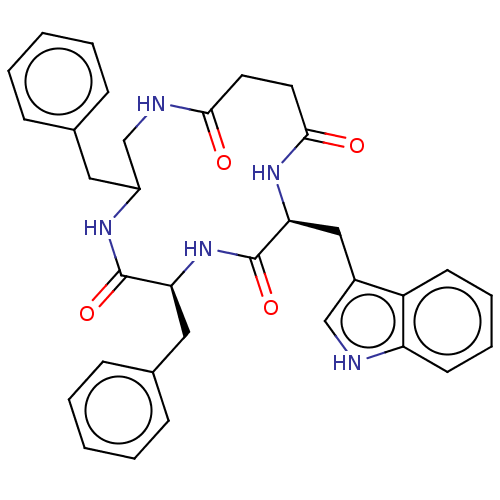 (CHEMBL131024) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Binding affinity towards Tachykinin receptor 2 | J Med Chem 43: 4041-4 (2000) Article DOI: 10.1021/jm0010217 BindingDB Entry DOI: 10.7270/Q2Z322CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50472909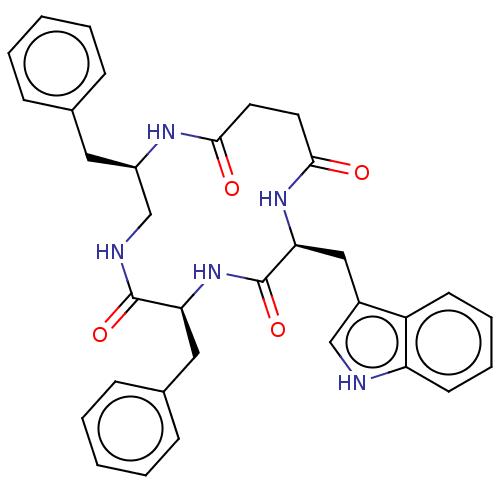 (CHEMBL2112669) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Binding affinity towards Tachykinin receptor 2 | J Med Chem 43: 4041-4 (2000) Article DOI: 10.1021/jm0010217 BindingDB Entry DOI: 10.7270/Q2Z322CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM21642 ((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046641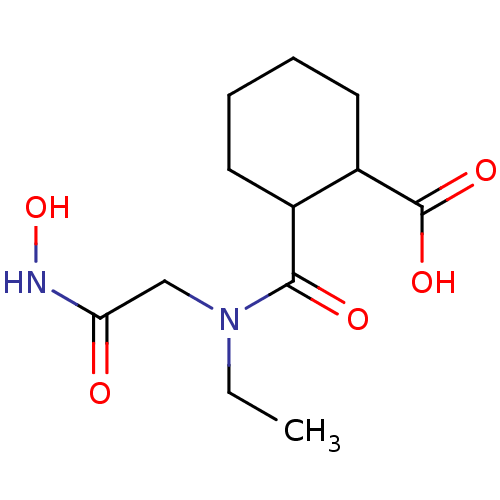 (1R,2R-trans-2-(Ethyl-hydroxycarbamoylmethyl-carbam...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070307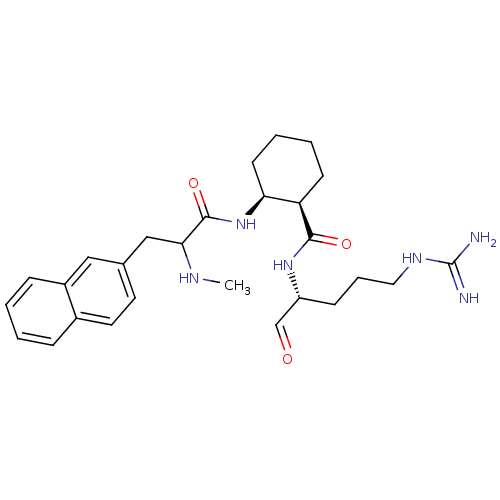 ((1R,2S)-2-(2-Methylamino-3-naphthalen-2-yl-propion...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of thrombin using the synthetic substrate chromozym TH | Bioorg Med Chem Lett 8: 1249-54 (1999) BindingDB Entry DOI: 10.7270/Q2FF3RH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046633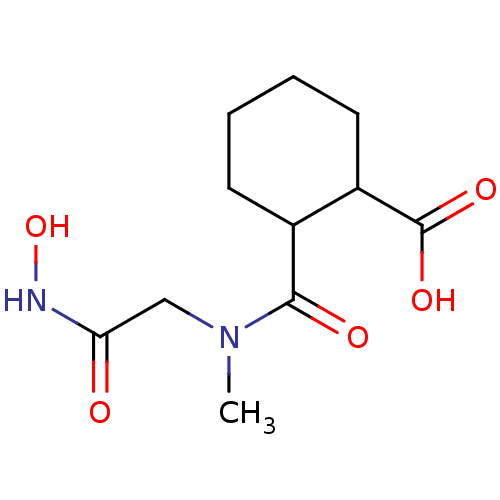 (1R,2R-trans-2-(Hydroxycarbamoylmethyl-methyl-carba...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046633 (1R,2R-trans-2-(Hydroxycarbamoylmethyl-methyl-carba...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50228863 ((S)-1-((R)-2-Methylamino-3-phenyl-propionyl)-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of thrombin using the synthetic substrate chromozym TH | Bioorg Med Chem Lett 8: 1249-54 (1999) BindingDB Entry DOI: 10.7270/Q2FF3RH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046632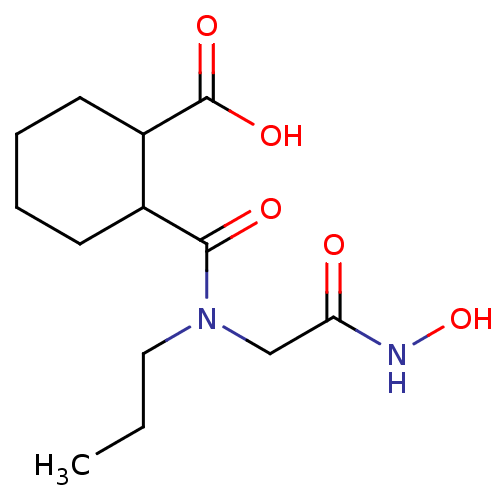 (CHEMBL159829 | Cis-2-(Hydroxycarbamoylmethyl-propy...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046632 (CHEMBL159829 | Cis-2-(Hydroxycarbamoylmethyl-propy...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046621 (CHEMBL352453 | Trans-2-(Hydroxycarbamoylmethyl-iso...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046629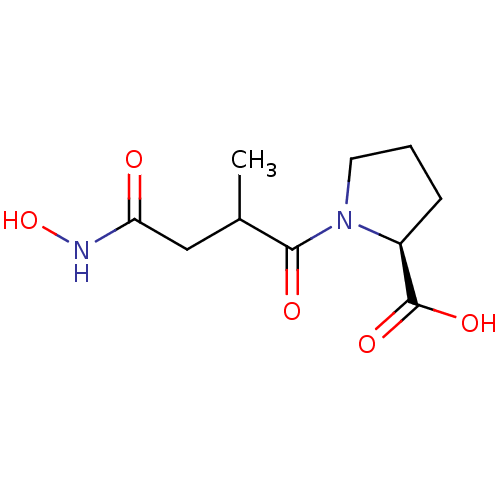 (1-(3-Hydroxycarbamoyl-2-methyl-propionyl)-pyrrolid...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046628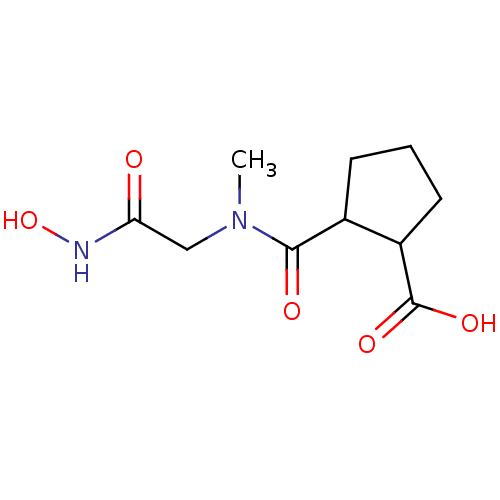 (CHEMBL434293 | Trans-2-(Hydroxycarbamoylmethyl-met...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046631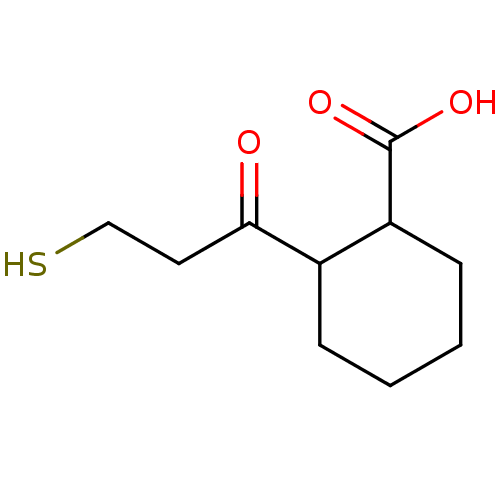 (CHEMBL159791 | Cis-2-(3-Mercapto-propionyl)-cycloh...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046631 (CHEMBL159791 | Cis-2-(3-Mercapto-propionyl)-cycloh...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046641 (1R,2R-trans-2-(Ethyl-hydroxycarbamoylmethyl-carbam...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046634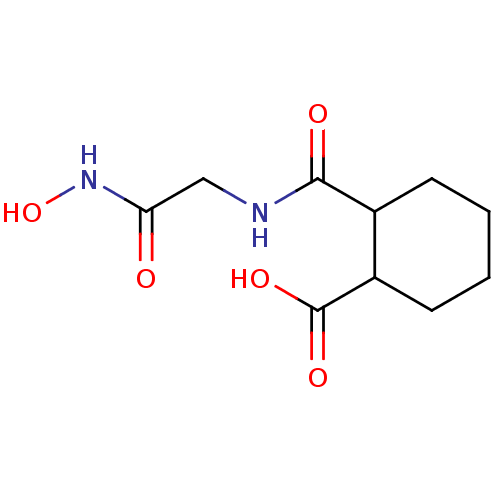 (CHEMBL161968 | Cis-2-(Hydroxycarbamoylmethyl-carba...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046634 (CHEMBL161968 | Cis-2-(Hydroxycarbamoylmethyl-carba...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070306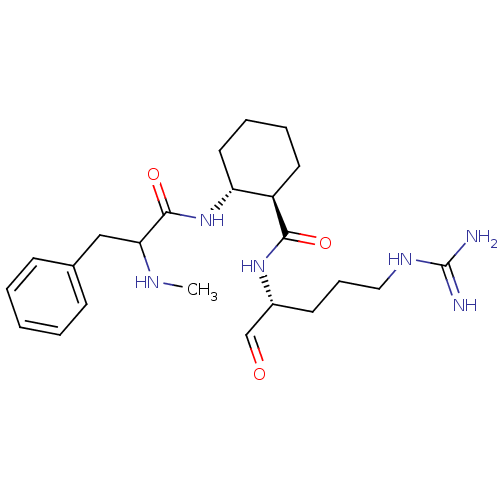 ((1R,2R)-2-(2-Methylamino-3-phenyl-propionylamino)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of thrombin using the synthetic substrate chromozym TH | Bioorg Med Chem Lett 8: 1249-54 (1999) BindingDB Entry DOI: 10.7270/Q2FF3RH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046638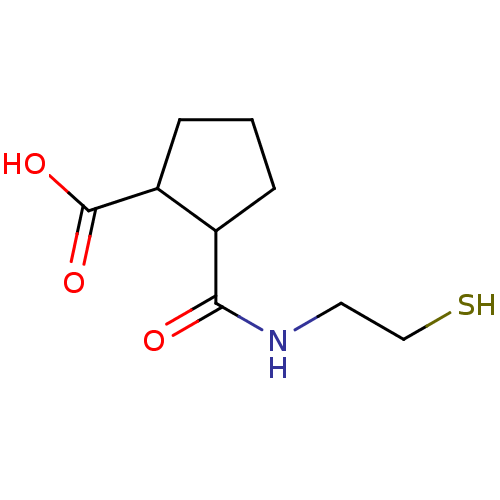 (CHEMBL163601 | Cis-2-(2-Mercapto-ethylcarbamoyl)-c...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070305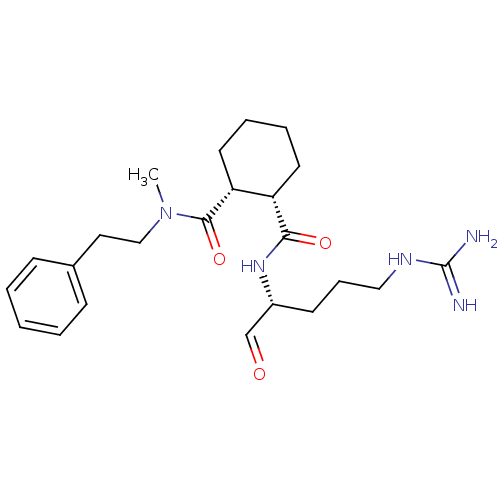 ((1R,2S)-Cyclohexane-1,2-dicarboxylic acid 1-[((R)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of thrombin using the synthetic substrate chromozym TH | Bioorg Med Chem Lett 8: 1249-54 (1999) BindingDB Entry DOI: 10.7270/Q2FF3RH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046630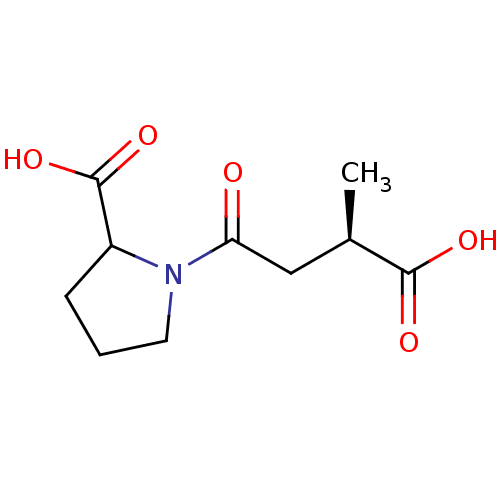 (1-(3-Carboxy-butyryl)-pyrrolidine-2-carboxylic aci...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046626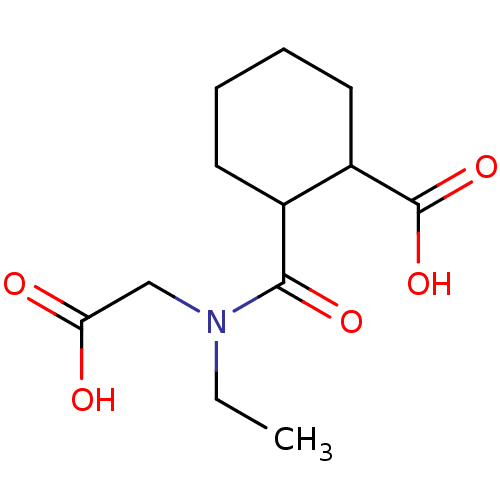 (CHEMBL163568 | Cis-2-(Carboxymethyl-ethyl-carbamoy...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046626 (CHEMBL163568 | Cis-2-(Carboxymethyl-ethyl-carbamoy...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070304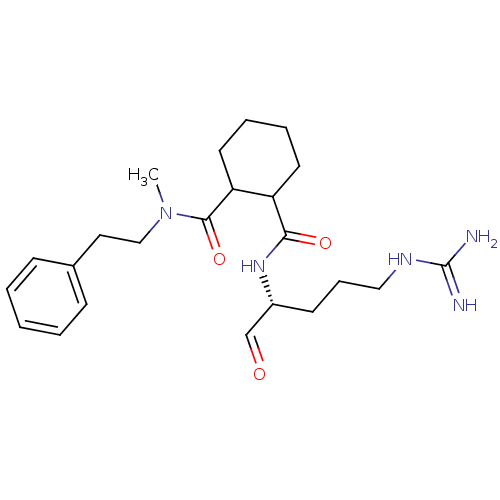 (CHEMBL281784 | Cyclohexane-1,2-dicarboxylic acid 1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of thrombin using the synthetic substrate chromozym TH | Bioorg Med Chem Lett 8: 1249-54 (1999) BindingDB Entry DOI: 10.7270/Q2FF3RH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046636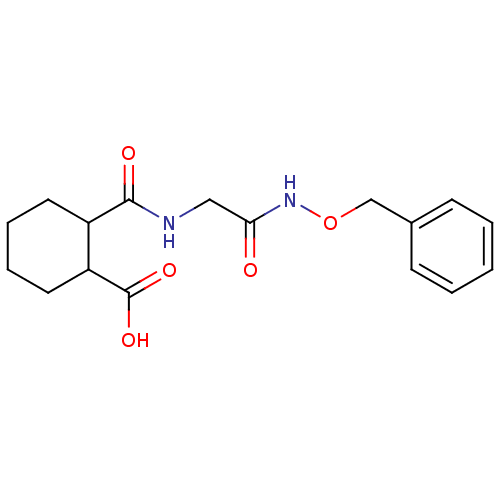 (CHEMBL159996 | Cis-2-[(Benzyloxycarbamoyl-methyl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046640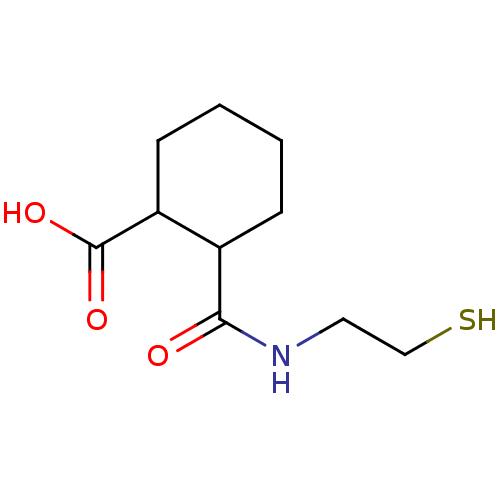 (CHEMBL163549 | Cis-2-(2-Mercapto-ethylcarbamoyl)-c...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046627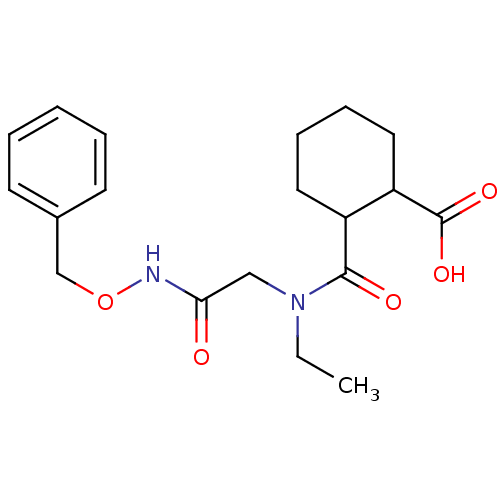 (CHEMBL161552 | Cis-2-[(Benzyloxycarbamoyl-methyl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046636 (CHEMBL159996 | Cis-2-[(Benzyloxycarbamoyl-methyl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046640 (CHEMBL163549 | Cis-2-(2-Mercapto-ethylcarbamoyl)-c...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046627 (CHEMBL161552 | Cis-2-[(Benzyloxycarbamoyl-methyl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046624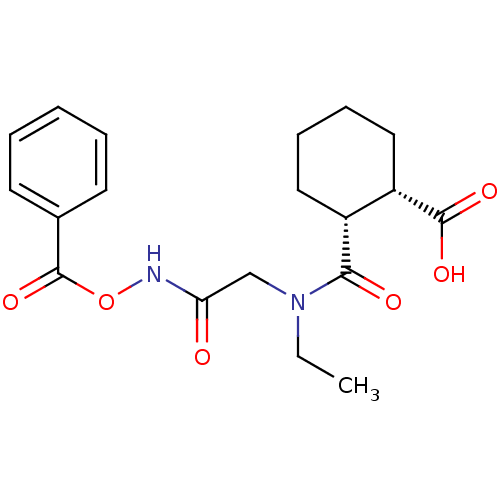 (1R,2R-trans-2-[(Benzoyloxycarbamoyl-methyl)-ethyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046620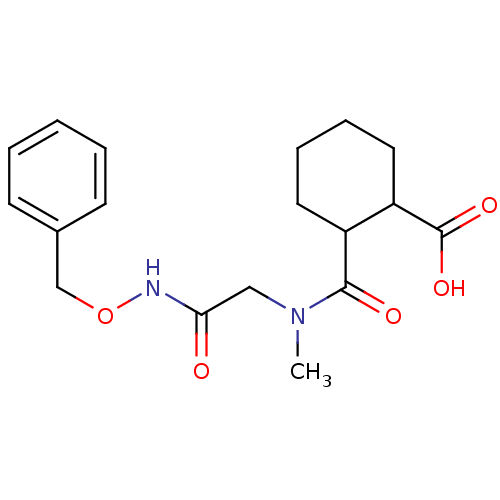 (CHEMBL159956 | Cis-2-[(Benzyloxycarbamoyl-methyl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046625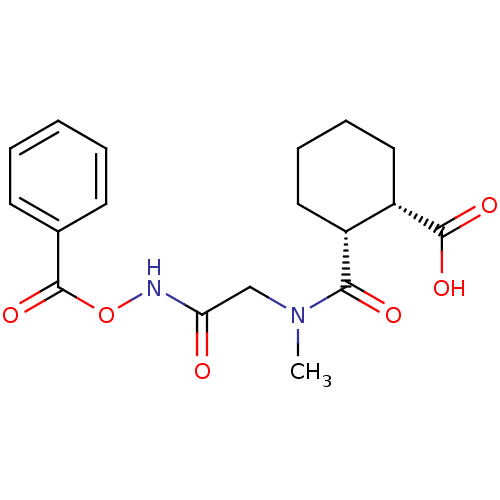 (1R,2R-trans-2-[(Benzoyloxycarbamoyl-methyl)-carbam...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046622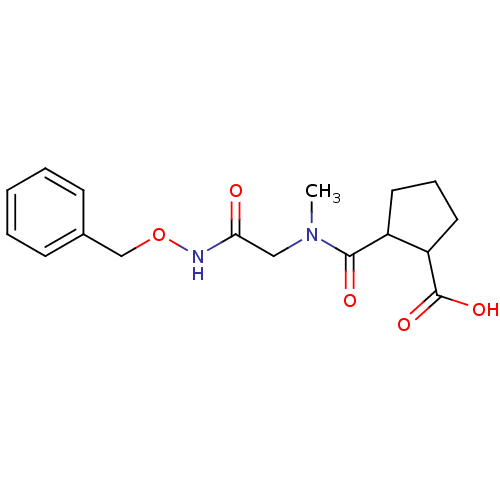 (CHEMBL159995 | Trans-2-[(Benzyloxycarbamoyl-methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046635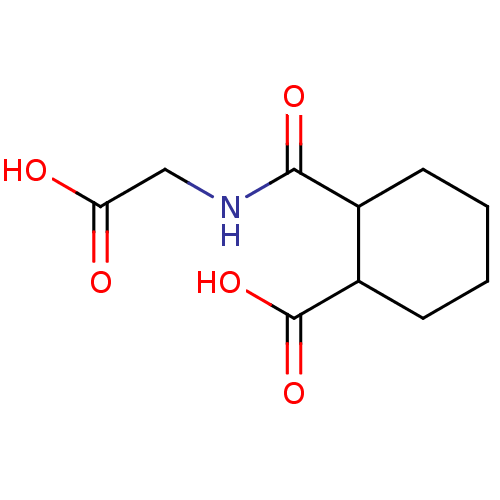 (CHEMBL159973 | Cis-2-(Carboxymethyl-carbamoyl)-cyc...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 73 total ) | Next | Last >> |