 Found 212 hits with Last Name = 'ducho' and Initial = 'c'
Found 212 hits with Last Name = 'ducho' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50037187
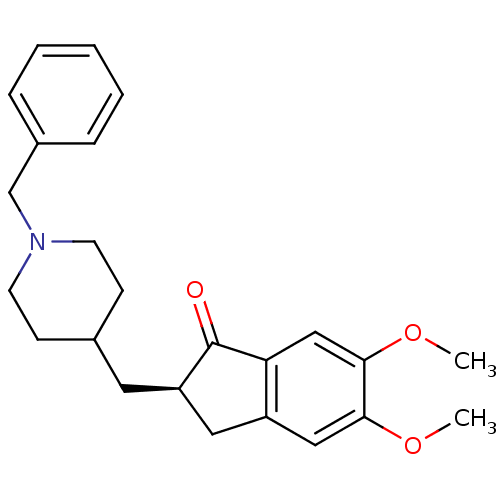
((2R)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimeth...)Show SMILES COc1cc2C[C@@H](CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3/t20-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Binding affinity tested against acetylcholinesterase in Torpedo californica |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50037176
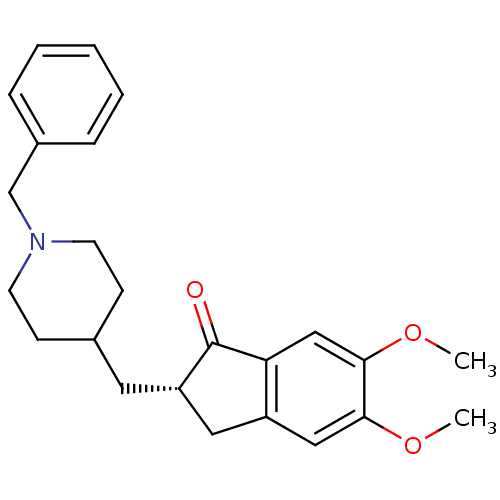
((2S)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimeth...)Show SMILES COc1cc2C[C@H](CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3/t20-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Binding affinity tested against acetylcholinesterase in Torpedo californica |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Staphylococcus aureus (strain MRSA252)) | BDBM50465326

(CHEMBL4283345)Show SMILES [H][C@](O[C@@H]1O[C@H](CN)[C@@H](O)[C@H]1OC)([C@H](NCCCNC(=O)[C@@H](NC(=O)[C@@H](NC(=O)N[C@@H](C(C)C)C(O)=O)[C@]1([H])CCNC(=N)N1)[C@@H](OC(=O)CCCCCCCCCCCCC(C)C)C(C)C)C(O)=O)[C@@]1([H])O[C@H]([C@H](O)[C@@H]1O)n1ccc(=O)[nH]c1=O |r| Show InChI InChI=1S/C54H93N11O18/c1-28(2)19-16-14-12-10-8-9-11-13-15-17-20-34(67)81-42(30(5)6)37(62-47(72)36(31-21-25-59-52(56)60-31)64-53(77)63-35(29(3)4)49(73)74)46(71)58-24-18-23-57-38(50(75)76)43(83-51-45(79-7)39(68)32(27-55)80-51)44-40(69)41(70)48(82-44)65-26-22-33(66)61-54(65)78/h22,26,28-32,35-45,48,51,57,68-70H,8-21,23-25,27,55H2,1-7H3,(H,58,71)(H,62,72)(H,73,74)(H,75,76)(H3,56,59,60)(H,61,66,78)(H2,63,64,77)/t31-,32+,35-,36-,37-,38-,39+,40-,41+,42-,43-,44-,45+,48+,51-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus MraY expressed in Escherichia coli membrane using Park's nucleotide as substrate pretreated for 30 mins followed ... |
J Nat Prod 81: 942-948 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01054
BindingDB Entry DOI: 10.7270/Q2XS5Z1V |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Aquifex aeolicus (strain VF5)) | BDBM50465326

(CHEMBL4283345)Show SMILES [H][C@](O[C@@H]1O[C@H](CN)[C@@H](O)[C@H]1OC)([C@H](NCCCNC(=O)[C@@H](NC(=O)[C@@H](NC(=O)N[C@@H](C(C)C)C(O)=O)[C@]1([H])CCNC(=N)N1)[C@@H](OC(=O)CCCCCCCCCCCCC(C)C)C(C)C)C(O)=O)[C@@]1([H])O[C@H]([C@H](O)[C@@H]1O)n1ccc(=O)[nH]c1=O |r| Show InChI InChI=1S/C54H93N11O18/c1-28(2)19-16-14-12-10-8-9-11-13-15-17-20-34(67)81-42(30(5)6)37(62-47(72)36(31-21-25-59-52(56)60-31)64-53(77)63-35(29(3)4)49(73)74)46(71)58-24-18-23-57-38(50(75)76)43(83-51-45(79-7)39(68)32(27-55)80-51)44-40(69)41(70)48(82-44)65-26-22-33(66)61-54(65)78/h22,26,28-32,35-45,48,51,57,68-70H,8-21,23-25,27,55H2,1-7H3,(H,58,71)(H,62,72)(H,73,74)(H,75,76)(H3,56,59,60)(H,61,66,78)(H2,63,64,77)/t31-,32+,35-,36-,37-,38-,39+,40-,41+,42-,43-,44-,45+,48+,51-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of Aquifex aeolicus MraY expressed in Escherichia coli C41(DE3) using Park's nucleotide as substrate pretreated for 30 mins followed by su... |
J Nat Prod 81: 942-948 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01054
BindingDB Entry DOI: 10.7270/Q2XS5Z1V |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Staphylococcus aureus (strain MRSA252)) | BDBM50465324
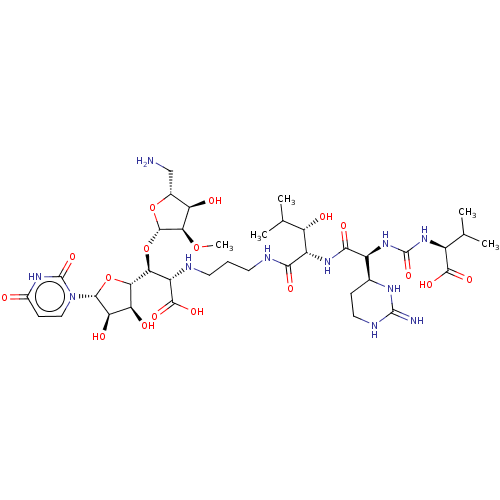
(CHEMBL4286766)Show SMILES [H][C@](O[C@@H]1O[C@H](CN)[C@@H](O)[C@H]1OC)([C@H](NCCCNC(=O)[C@@H](NC(=O)[C@@H](NC(=O)N[C@@H](C(C)C)C(O)=O)[C@]1([H])CCNC(=N)N1)[C@@H](O)C(C)C)C(O)=O)[C@@]1([H])O[C@H]([C@H](O)[C@@H]1O)n1ccc(=O)[nH]c1=O |r| Show InChI InChI=1S/C38H63N11O17/c1-14(2)19(33(57)58)47-37(61)48-20(16-7-11-43-36(40)44-16)31(56)46-21(23(51)15(3)4)30(55)42-10-6-9-41-22(34(59)60)27(66-35-29(63-5)24(52)17(13-39)64-35)28-25(53)26(54)32(65-28)49-12-8-18(50)45-38(49)62/h8,12,14-17,19-29,32,35,41,51-54H,6-7,9-11,13,39H2,1-5H3,(H,42,55)(H,46,56)(H,57,58)(H,59,60)(H3,40,43,44)(H,45,50,62)(H2,47,48,61)/t16-,17+,19-,20-,21-,22-,23-,24+,25-,26+,27-,28-,29+,32+,35-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus MraY expressed in Escherichia coli membrane using Park's nucleotide as substrate pretreated for 30 mins followed ... |
J Nat Prod 81: 942-948 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01054
BindingDB Entry DOI: 10.7270/Q2XS5Z1V |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Staphylococcus aureus (strain MRSA252)) | BDBM50465327
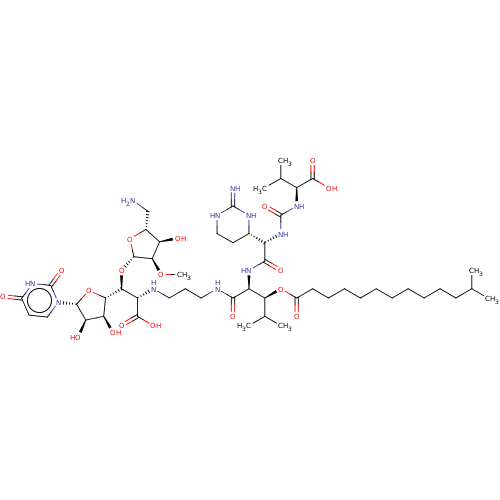
(CHEMBL4279378)Show SMILES [H][C@](O[C@@H]1O[C@H](CN)[C@@H](O)[C@H]1OC)([C@H](NCCCNC(=O)[C@@H](NC(=O)[C@@H](NC(=O)N[C@@H](C(C)C)C(O)=O)[C@]1([H])CCNC(=N)N1)[C@@H](OC(=O)CCCCCCCCCCC(C)C)C(C)C)C(O)=O)[C@@]1([H])O[C@H]([C@H](O)[C@@H]1O)n1ccc(=O)[nH]c1=O |r| Show InChI InChI=1S/C52H89N11O18/c1-26(2)17-14-12-10-8-9-11-13-15-18-32(65)79-40(28(5)6)35(60-45(70)34(29-19-23-57-50(54)58-29)62-51(75)61-33(27(3)4)47(71)72)44(69)56-22-16-21-55-36(48(73)74)41(81-49-43(77-7)37(66)30(25-53)78-49)42-38(67)39(68)46(80-42)63-24-20-31(64)59-52(63)76/h20,24,26-30,33-43,46,49,55,66-68H,8-19,21-23,25,53H2,1-7H3,(H,56,69)(H,60,70)(H,71,72)(H,73,74)(H3,54,57,58)(H,59,64,76)(H2,61,62,75)/t29-,30+,33-,34-,35-,36-,37+,38-,39+,40-,41-,42-,43+,46+,49-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus MraY expressed in Escherichia coli membrane using Park's nucleotide as substrate pretreated for 30 mins followed ... |
J Nat Prod 81: 942-948 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01054
BindingDB Entry DOI: 10.7270/Q2XS5Z1V |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Staphylococcus aureus (strain MRSA252)) | BDBM50465325

(CHEMBL4284923)Show SMILES [H][C@](O[C@@H]1O[C@H](CNC(C)=O)[C@@H](O)[C@H]1OC)([C@H](NCCCNC(=O)[C@@H](NC(=O)[C@@H](NC(=O)N[C@@H](C(C)C)C(O)=O)[C@]1([H])CCNC(=N)N1)[C@@H](O)C(C)C)C(O)=O)[C@@]1([H])O[C@H]([C@H](O)[C@@H]1O)n1ccc(=O)[nH]c1=O |r| Show InChI InChI=1S/C40H65N11O18/c1-15(2)21(35(60)61)49-39(64)50-22(18-8-12-44-38(41)46-18)33(59)48-23(25(54)16(3)4)32(58)43-11-7-10-42-24(36(62)63)29(69-37-31(66-6)26(55)19(67-37)14-45-17(5)52)30-27(56)28(57)34(68-30)51-13-9-20(53)47-40(51)65/h9,13,15-16,18-19,21-31,34,37,42,54-57H,7-8,10-12,14H2,1-6H3,(H,43,58)(H,45,52)(H,48,59)(H,60,61)(H,62,63)(H3,41,44,46)(H,47,53,65)(H2,49,50,64)/t18-,19+,21-,22-,23-,24-,25-,26+,27-,28+,29-,30-,31+,34+,37-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0930 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus MraY expressed in Escherichia coli membrane using Park's nucleotide as substrate pretreated for 30 mins followed ... |
J Nat Prod 81: 942-948 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01054
BindingDB Entry DOI: 10.7270/Q2XS5Z1V |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Aquifex aeolicus (strain VF5)) | BDBM50465327

(CHEMBL4279378)Show SMILES [H][C@](O[C@@H]1O[C@H](CN)[C@@H](O)[C@H]1OC)([C@H](NCCCNC(=O)[C@@H](NC(=O)[C@@H](NC(=O)N[C@@H](C(C)C)C(O)=O)[C@]1([H])CCNC(=N)N1)[C@@H](OC(=O)CCCCCCCCCCC(C)C)C(C)C)C(O)=O)[C@@]1([H])O[C@H]([C@H](O)[C@@H]1O)n1ccc(=O)[nH]c1=O |r| Show InChI InChI=1S/C52H89N11O18/c1-26(2)17-14-12-10-8-9-11-13-15-18-32(65)79-40(28(5)6)35(60-45(70)34(29-19-23-57-50(54)58-29)62-51(75)61-33(27(3)4)47(71)72)44(69)56-22-16-21-55-36(48(73)74)41(81-49-43(77-7)37(66)30(25-53)78-49)42-38(67)39(68)46(80-42)63-24-20-31(64)59-52(63)76/h20,24,26-30,33-43,46,49,55,66-68H,8-19,21-23,25,53H2,1-7H3,(H,56,69)(H,60,70)(H,71,72)(H,73,74)(H3,54,57,58)(H,59,64,76)(H2,61,62,75)/t29-,30+,33-,34-,35-,36-,37+,38-,39+,40-,41-,42-,43+,46+,49-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of Aquifex aeolicus MraY expressed in Escherichia coli C41(DE3) using Park's nucleotide as substrate pretreated for 30 mins followed by su... |
J Nat Prod 81: 942-948 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01054
BindingDB Entry DOI: 10.7270/Q2XS5Z1V |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Aquifex aeolicus (strain VF5)) | BDBM50465324

(CHEMBL4286766)Show SMILES [H][C@](O[C@@H]1O[C@H](CN)[C@@H](O)[C@H]1OC)([C@H](NCCCNC(=O)[C@@H](NC(=O)[C@@H](NC(=O)N[C@@H](C(C)C)C(O)=O)[C@]1([H])CCNC(=N)N1)[C@@H](O)C(C)C)C(O)=O)[C@@]1([H])O[C@H]([C@H](O)[C@@H]1O)n1ccc(=O)[nH]c1=O |r| Show InChI InChI=1S/C38H63N11O17/c1-14(2)19(33(57)58)47-37(61)48-20(16-7-11-43-36(40)44-16)31(56)46-21(23(51)15(3)4)30(55)42-10-6-9-41-22(34(59)60)27(66-35-29(63-5)24(52)17(13-39)64-35)28-25(53)26(54)32(65-28)49-12-8-18(50)45-38(49)62/h8,12,14-17,19-29,32,35,41,51-54H,6-7,9-11,13,39H2,1-5H3,(H,42,55)(H,46,56)(H,57,58)(H,59,60)(H3,40,43,44)(H,45,50,62)(H2,47,48,61)/t16-,17+,19-,20-,21-,22-,23-,24+,25-,26+,27-,28-,29+,32+,35-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of Aquifex aeolicus MraY expressed in Escherichia coli C41(DE3) using Park's nucleotide as substrate pretreated for 30 mins followed by su... |
J Nat Prod 81: 942-948 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01054
BindingDB Entry DOI: 10.7270/Q2XS5Z1V |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Aquifex aeolicus (strain VF5)) | BDBM50465325

(CHEMBL4284923)Show SMILES [H][C@](O[C@@H]1O[C@H](CNC(C)=O)[C@@H](O)[C@H]1OC)([C@H](NCCCNC(=O)[C@@H](NC(=O)[C@@H](NC(=O)N[C@@H](C(C)C)C(O)=O)[C@]1([H])CCNC(=N)N1)[C@@H](O)C(C)C)C(O)=O)[C@@]1([H])O[C@H]([C@H](O)[C@@H]1O)n1ccc(=O)[nH]c1=O |r| Show InChI InChI=1S/C40H65N11O18/c1-15(2)21(35(60)61)49-39(64)50-22(18-8-12-44-38(41)46-18)33(59)48-23(25(54)16(3)4)32(58)43-11-7-10-42-24(36(62)63)29(69-37-31(66-6)26(55)19(67-37)14-45-17(5)52)30-27(56)28(57)34(68-30)51-13-9-20(53)47-40(51)65/h9,13,15-16,18-19,21-31,34,37,42,54-57H,7-8,10-12,14H2,1-6H3,(H,43,58)(H,45,52)(H,48,59)(H,60,61)(H,62,63)(H3,41,44,46)(H,47,53,65)(H2,49,50,64)/t18-,19+,21-,22-,23-,24-,25-,26+,27-,28+,29-,30-,31+,34+,37-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of Aquifex aeolicus MraY expressed in Escherichia coli C41(DE3) using Park's nucleotide as substrate pretreated for 30 mins followed by su... |
J Nat Prod 81: 942-948 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01054
BindingDB Entry DOI: 10.7270/Q2XS5Z1V |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50465328

(CHEMBL4283943)Show SMILES [H][C@](O[C@@H]1O[C@H](CN)[C@@H](O)[C@H]1OC)([C@H](NCCCNC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)N[C@@H](C(C)C)C(O)=O)[C@]1([H])CCNC(=N)N1)C(O)=O)[C@@]1([H])O[C@H]([C@H](O)[C@@H]1O)n1ccc(=O)[nH]c1=O |r| Show InChI InChI=1S/C38H63N11O16/c1-15(2)13-18(44-31(55)22(17-7-11-43-36(40)45-17)48-37(60)47-21(16(3)4)33(56)57)30(54)42-10-6-9-41-23(34(58)59)27(65-35-29(62-5)24(51)19(14-39)63-35)28-25(52)26(53)32(64-28)49-12-8-20(50)46-38(49)61/h8,12,15-19,21-29,32,35,41,51-53H,6-7,9-11,13-14,39H2,1-5H3,(H,42,54)(H,44,55)(H,56,57)(H,58,59)(H3,40,43,45)(H,46,50,61)(H2,47,48,60)/t17-,18-,19+,21-,22-,23-,24+,25-,26+,27-,28-,29+,32+,35-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis H37Rv MraY expressed in Mycobacterium smegmatis using Park's nucleotide-N-epsilon-C6-dansyl as substrate mea... |
J Nat Prod 81: 942-948 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01054
BindingDB Entry DOI: 10.7270/Q2XS5Z1V |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50409820

(CHEMBL2114386)Show SMILES Cc1cccc2CO[P@](=O)(OC[C@H]3O[C@H](C=C3)n3cc(C)c(=O)[nH]c3=O)Oc12 |r,c:15| Show InChI InChI=1S/C18H19N2O7P/c1-11-4-3-5-13-9-24-28(23,27-16(11)13)25-10-14-6-7-15(26-14)20-8-12(2)17(21)19-18(20)22/h3-8,14-15H,9-10H2,1-2H3,(H,19,21,22)/t14-,15+,28+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50146850
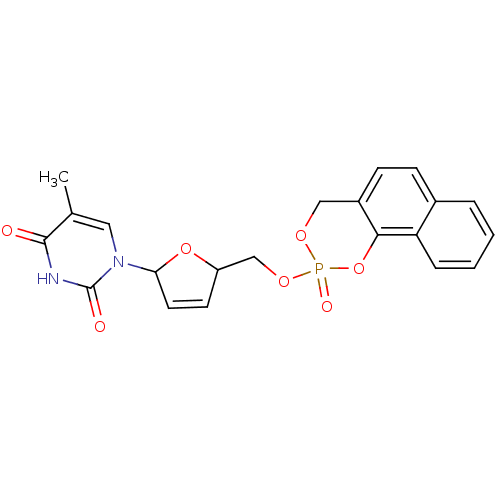
(5-Methyl-1-[5-(3-oxo-1H-2,4-dioxa-3lambda*5*-phosp...)Show SMILES Cc1cn(C2OC(COP3(=O)OCc4ccc5ccccc5c4O3)C=C2)c(=O)[nH]c1=O |c:27| Show InChI InChI=1S/C21H19N2O7P/c1-13-10-23(21(25)22-20(13)24)18-9-8-16(29-18)12-28-31(26)27-11-15-7-6-14-4-2-3-5-17(14)19(15)30-31/h2-10,16,18H,11-12H2,1H3,(H,22,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50146849
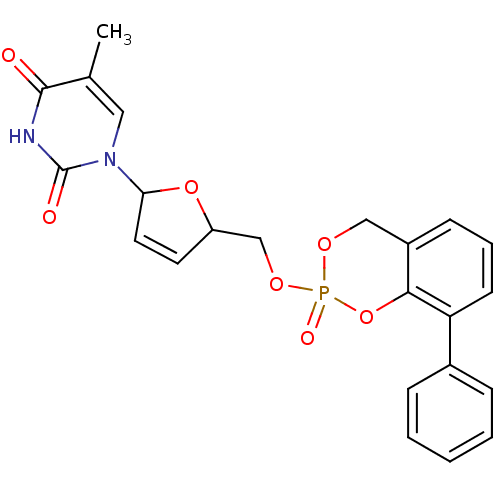
(5-Methyl-1-[5-(2-oxo-8-phenyl-4H-2lambda*5*-benzo[...)Show SMILES Cc1cn(C2OC(COP3(=O)OCc4cccc(-c5ccccc5)c4O3)C=C2)c(=O)[nH]c1=O |c:29| Show InChI InChI=1S/C23H21N2O7P/c1-15-12-25(23(27)24-22(15)26)20-11-10-18(31-20)14-30-33(28)29-13-17-8-5-9-19(21(17)32-33)16-6-3-2-4-7-16/h2-12,18,20H,13-14H2,1H3,(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50206640
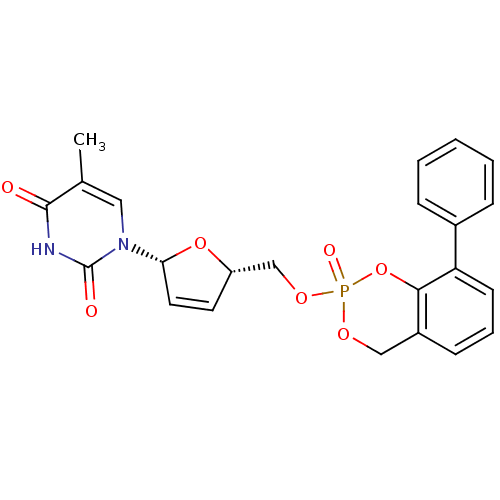
(3-phenyl-cyclosal-d4TMP | 5-Methyl-1-[(2R,5S)-5-(2...)Show SMILES Cc1cn([C@@H]2O[C@H](COP3(=O)OCc4cccc(-c5ccccc5)c4O3)C=C2)c(=O)[nH]c1=O |r,c:29| Show InChI InChI=1S/C23H21N2O7P/c1-15-12-25(23(27)24-22(15)26)20-11-10-18(31-20)14-30-33(28)29-13-17-8-5-9-19(21(17)32-33)16-6-3-2-4-7-16/h2-12,18,20H,13-14H2,1H3,(H,24,26,27)/t18-,20+,33?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE |
J Med Chem 50: 1335-46 (2007)
Article DOI: 10.1021/jm0611713
BindingDB Entry DOI: 10.7270/Q25H7H3J |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50146859

(5-Methyl-1-[5-(2-oxo-4H-1,3-dioxa-2lambda*5*-phosp...)Show SMILES Cc1cn(C2OC(COP3(=O)OCc4cc5ccccc5cc4O3)C=C2)c(=O)[nH]c1=O |c:27| Show InChI InChI=1S/C21H19N2O7P/c1-13-10-23(21(25)22-20(13)24)19-7-6-17(29-19)12-28-31(26)27-11-16-8-14-4-2-3-5-15(14)9-18(16)30-31/h2-10,17,19H,11-12H2,1H3,(H,22,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50146881

(1-[5-(5-Chloro-2-oxo-4H-2lambda*5*-benzo[1,3,2]dio...)Show SMILES Cc1cn(C2OC(COP3(=O)OCc4c(Cl)cccc4O3)C=C2)c(=O)[nH]c1=O |c:23| Show InChI InChI=1S/C17H16ClN2O7P/c1-10-7-20(17(22)19-16(10)21)15-6-5-11(26-15)8-24-28(23)25-9-12-13(18)3-2-4-14(12)27-28/h2-7,11,15H,8-9H2,1H3,(H,19,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50063918

((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-1-methyl...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)[C@H](CSc1cccs1)C(=O)NO Show InChI InChI=1S/C23H31N3O4S2/c1-15(2)12-17(18(22(28)26-30)14-32-20-10-7-11-31-20)21(27)25-19(23(29)24-3)13-16-8-5-4-6-9-16/h4-11,15,17-19,30H,12-14H2,1-3H3,(H,24,29)(H,25,27)(H,26,28)/t17-,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00187f
BindingDB Entry DOI: 10.7270/Q2N01BG9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM50064050
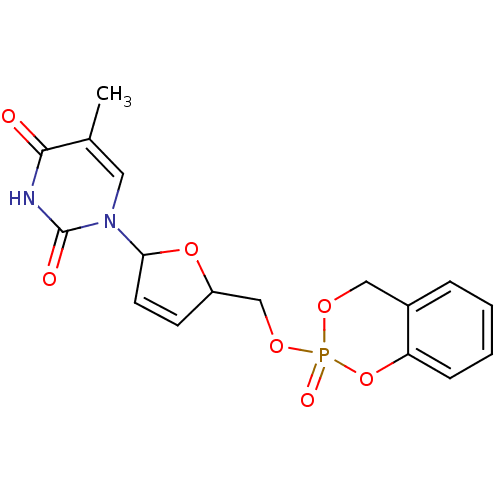
(5-Methyl-1-[(2R,5S)-5-(2-oxo-4H-2lambda*5*-benzo[1...)Show SMILES Cc1cn(C2OC(COP3(=O)OCc4ccccc4O3)C=C2)c(=O)[nH]c1=O |c:22| Show InChI InChI=1S/C17H17N2O7P/c1-11-8-19(17(21)18-16(11)20)15-7-6-13(25-15)10-24-27(22)23-9-12-4-2-3-5-14(12)26-27/h2-8,13,15H,9-10H2,1H3,(H,18,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50206637
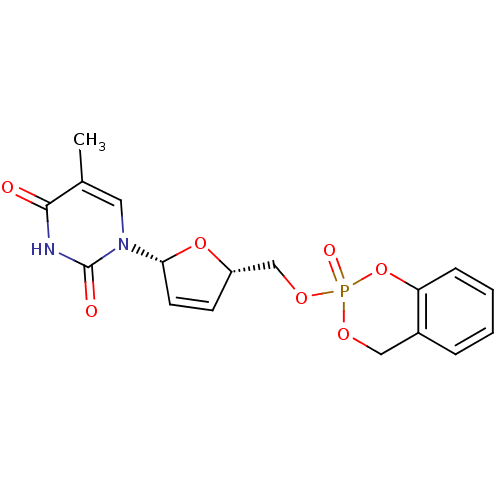
(5-methyl-1-[(2R,5R)-5-(2-oxo-4H-2lambda5-benzo[1,3...)Show SMILES Cc1cn([C@@H]2O[C@H](COP3(=O)OCc4ccccc4O3)C=C2)c(=O)[nH]c1=O |r,c:22| Show InChI InChI=1S/C17H17N2O7P/c1-11-8-19(17(21)18-16(11)20)15-7-6-13(25-15)10-24-27(22)23-9-12-4-2-3-5-14(12)26-27/h2-8,13,15H,9-10H2,1H3,(H,18,20,21)/t13-,15+,27?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE |
J Med Chem 50: 1335-46 (2007)
Article DOI: 10.1021/jm0611713
BindingDB Entry DOI: 10.7270/Q25H7H3J |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50206638
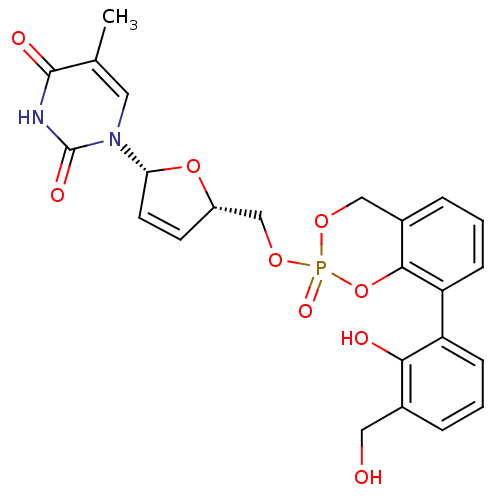
((Rp)-3-sal-cyclosal-d4TMP | (Sp)-3-sal-cyclosal-d4...)Show SMILES Cc1cn([C@@H]2O[C@H](COP3(=O)OCc4cccc(c4O3)-c3cccc(CO)c3O)C=C2)c(=O)[nH]c1=O |c:32| Show InChI InChI=1S/C24H23N2O9P/c1-14-10-26(24(30)25-23(14)29)20-9-8-17(34-20)13-33-36(31)32-12-16-5-3-7-19(22(16)35-36)18-6-2-4-15(11-27)21(18)28/h2-10,17,20,27-28H,11-13H2,1H3,(H,25,29,30)/t17-,20+,36?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE |
J Med Chem 50: 1335-46 (2007)
Article DOI: 10.1021/jm0611713
BindingDB Entry DOI: 10.7270/Q25H7H3J |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50076440
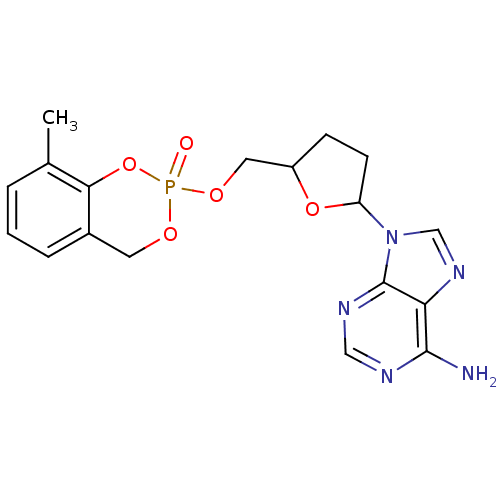
(9-[5-(8-Methyl-2-oxo-4H-2lambda*5*-benzo[1,3,2]dio...)Show SMILES Cc1cccc2COP(=O)(OCC3CCC(O3)n3cnc4c(N)ncnc34)Oc12 Show InChI InChI=1S/C18H20N5O5P/c1-11-3-2-4-12-7-25-29(24,28-16(11)12)26-8-13-5-6-14(27-13)23-10-22-15-17(19)20-9-21-18(15)23/h2-4,9-10,13-14H,5-8H2,1H3,(H2,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50146872
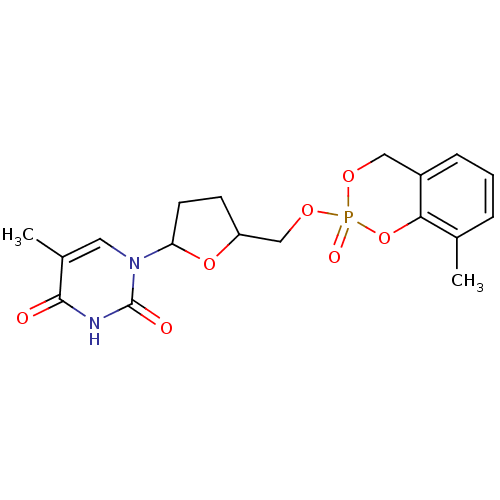
(5-Methyl-1-[5-(8-methyl-2-oxo-4H-2lambda*5*-benzo[...)Show SMILES Cc1cccc2COP(=O)(OCC3CCC(O3)n3cc(C)c(=O)[nH]c3=O)Oc12 Show InChI InChI=1S/C18H21N2O7P/c1-11-4-3-5-13-9-24-28(23,27-16(11)13)25-10-14-6-7-15(26-14)20-8-12(2)17(21)19-18(20)22/h3-5,8,14-15H,6-7,9-10H2,1-2H3,(H,19,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50422510
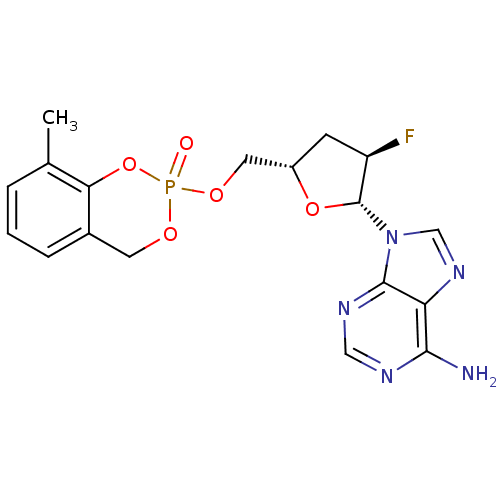
(CHEMBL2112391)Show SMILES Cc1cccc2COP(=O)(OC[C@@H]3C[C@@H](F)[C@@H](O3)n3cnc4c(N)ncnc34)Oc12 |r| Show InChI InChI=1S/C18H19FN5O5P/c1-10-3-2-4-11-6-26-30(25,29-15(10)11)27-7-12-5-13(19)18(28-12)24-9-23-14-16(20)21-8-22-17(14)24/h2-4,8-9,12-13,18H,5-7H2,1H3,(H2,20,21,22)/t12-,13+,18+,30?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50146867

(9-[5-(8-Methyl-2-oxo-4H-2lambda*5*-benzo[1,3,2]dio...)Show SMILES Cc1cccc2COP(=O)(OCC3OC(C=C3)n3cnc4c(N)ncnc34)Oc12 |c:15| Show InChI InChI=1S/C18H18N5O5P/c1-11-3-2-4-12-7-25-29(24,28-16(11)12)26-8-13-5-6-14(27-13)23-10-22-15-17(19)20-9-21-18(15)23/h2-6,9-10,13-14H,7-8H2,1H3,(H2,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50409820

(CHEMBL2114386)Show SMILES Cc1cccc2CO[P@](=O)(OC[C@H]3O[C@H](C=C3)n3cc(C)c(=O)[nH]c3=O)Oc12 |r,c:15| Show InChI InChI=1S/C18H19N2O7P/c1-11-4-3-5-13-9-24-28(23,27-16(11)13)25-10-14-6-7-15(26-14)20-8-12(2)17(21)19-18(20)22/h3-8,14-15H,9-10H2,1-2H3,(H,19,21,22)/t14-,15+,28+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50146868

((R)-N*6*-Cyclopropyl-9-[(R)-4-(8-methyl-2-oxo-4H-2...)Show SMILES Cc1cccc2COP(=O)(OC[C@@H]3CC(C=C3)n3cnc4c(NC5CC5)nc(N)nc34)Oc12 |c:15| Show InChI InChI=1S/C22H25N6O4P/c1-13-3-2-4-15-11-31-33(29,32-19(13)15)30-10-14-5-8-17(9-14)28-12-24-18-20(25-16-6-7-16)26-22(23)27-21(18)28/h2-5,8,12,14,16-17H,6-7,9-11H2,1H3,(H3,23,25,26,27)/t14-,17?,33?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Protein cereblon
(Homo sapiens (Human)) | BDBM50570884
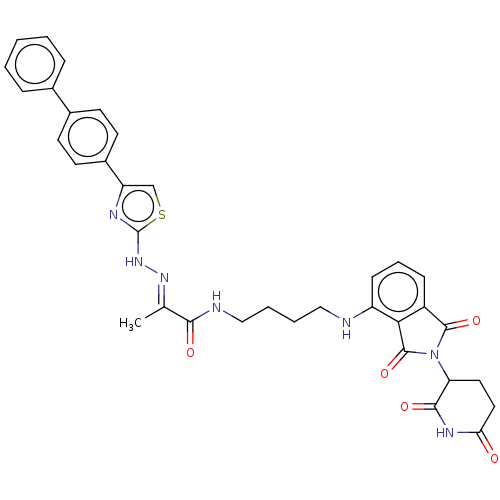
(CHEMBL4860669)Show SMILES C\C(=N/Nc1nc(cs1)-c1ccc(cc1)-c1ccccc1)C(=O)NCCCCNc1cccc2C(=O)N(C3CCC(=O)NC3=O)C(=O)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to Cereblon in human Flp293T cells transfected with BRD4(BD2)-GFP fusion protein and mCherry reporter assessed as inhibition of dBET... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113435
BindingDB Entry DOI: 10.7270/Q2QV3R91 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50146870

(1-[5-(5-Chloro-4-methyl-2-oxo-4H-2lambda*5*-benzo[...)Show SMILES CC1OP(=O)(OCC2OC(C=C2)n2cc(C)c(=O)[nH]c2=O)Oc2cccc(Cl)c12 |c:10| Show InChI InChI=1S/C18H18ClN2O7P/c1-10-8-21(18(23)20-17(10)22)15-7-6-12(26-15)9-25-29(24)27-11(2)16-13(19)4-3-5-14(16)28-29/h3-8,11-12,15H,9H2,1-2H3,(H,20,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50422509
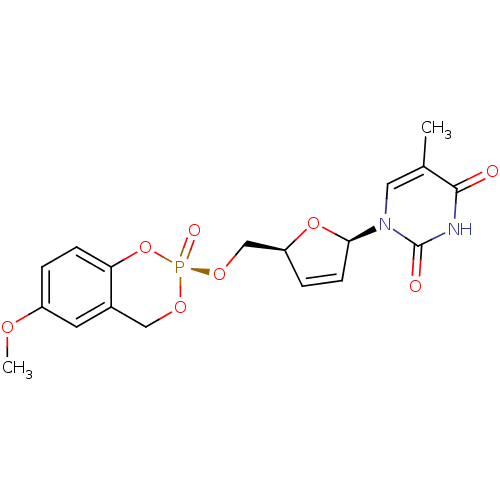
(CHEMBL2114383)Show SMILES COc1ccc2O[P@@](=O)(OC[C@H]3O[C@H](C=C3)n3cc(C)c(=O)[nH]c3=O)OCc2c1 |r,c:14| Show InChI InChI=1S/C18H19N2O8P/c1-11-8-20(18(22)19-17(11)21)16-6-4-14(27-16)10-26-29(23)25-9-12-7-13(24-2)3-5-15(12)28-29/h3-8,14,16H,9-10H2,1-2H3,(H,19,21,22)/t14-,16+,29+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50063918

((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-1-methyl...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)[C@H](CSc1cccs1)C(=O)NO Show InChI InChI=1S/C23H31N3O4S2/c1-15(2)12-17(18(22(28)26-30)14-32-20-10-7-11-31-20)21(27)25-19(23(29)24-3)13-16-8-5-4-6-9-16/h4-11,15,17-19,30H,12-14H2,1-3H3,(H,24,29)(H,25,27)(H,26,28)/t17-,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00187f
BindingDB Entry DOI: 10.7270/Q2N01BG9 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50146865
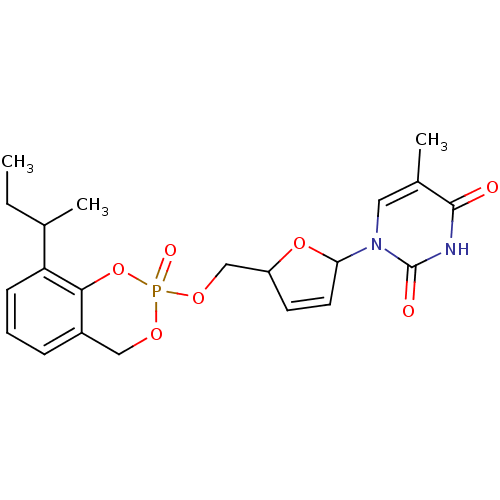
(1-[5-(8-sec-Butyl-2-oxo-4H-2lambda*5*-benzo[1,3,2]...)Show SMILES CCC(C)c1cccc2COP(=O)(OCC3OC(C=C3)n3cc(C)c(=O)[nH]c3=O)Oc12 |c:18| Show InChI InChI=1S/C21H25N2O7P/c1-4-13(2)17-7-5-6-15-11-27-31(26,30-19(15)17)28-12-16-8-9-18(29-16)23-10-14(3)20(24)22-21(23)25/h5-10,13,16,18H,4,11-12H2,1-3H3,(H,22,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50063918

((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-1-methyl...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)[C@H](CSc1cccs1)C(=O)NO Show InChI InChI=1S/C23H31N3O4S2/c1-15(2)12-17(18(22(28)26-30)14-32-20-10-7-11-31-20)21(27)25-19(23(29)24-3)13-16-8-5-4-6-9-16/h4-11,15,17-19,30H,12-14H2,1-3H3,(H,24,29)(H,25,27)(H,26,28)/t17-,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00187f
BindingDB Entry DOI: 10.7270/Q2N01BG9 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50206636
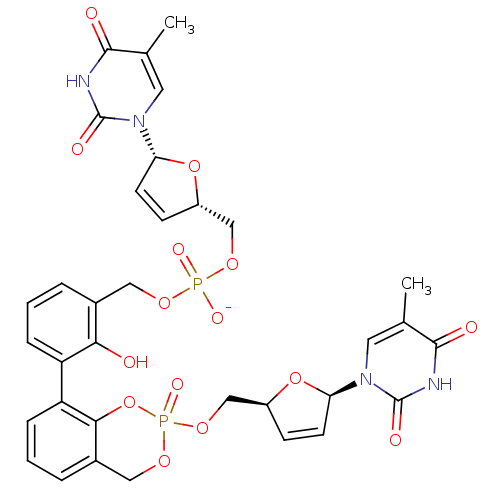
(CID44421019 | [2-hydroxy-3-(2-{[(2S,5R)-5-(5-methy...)Show SMILES Cc1cn([C@@H]2O[C@H](COP([O-])(=O)OCc3cccc(c3O)-c3cccc4COP(=O)(OC[C@H]5O[C@H](C=C5)n5cc(C)c(=O)[nH]c5=O)Oc34)C=C2)c(=O)[nH]c1=O |c:36,53| Show InChI InChI=1S/C34H34N4O15P2/c1-19-13-37(33(42)35-31(19)40)27-11-9-23(51-27)17-48-54(44,45)47-15-21-5-3-7-25(29(21)39)26-8-4-6-22-16-49-55(46,53-30(22)26)50-18-24-10-12-28(52-24)38-14-20(2)32(41)36-34(38)43/h3-14,23-24,27-28,39H,15-18H2,1-2H3,(H,44,45)(H,35,40,42)(H,36,41,43)/p-1/t23-,24-,27+,28+,55?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE |
J Med Chem 50: 1335-46 (2007)
Article DOI: 10.1021/jm0611713
BindingDB Entry DOI: 10.7270/Q25H7H3J |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50206638

((Rp)-3-sal-cyclosal-d4TMP | (Sp)-3-sal-cyclosal-d4...)Show SMILES Cc1cn([C@@H]2O[C@H](COP3(=O)OCc4cccc(c4O3)-c3cccc(CO)c3O)C=C2)c(=O)[nH]c1=O |c:32| Show InChI InChI=1S/C24H23N2O9P/c1-14-10-26(24(30)25-23(14)29)20-9-8-17(34-20)13-33-36(31)32-12-16-5-3-7-19(22(16)35-36)18-6-2-4-15(11-27)21(18)28/h2-10,17,20,27-28H,11-13H2,1H3,(H,25,29,30)/t17-,20+,36?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE |
J Med Chem 50: 1335-46 (2007)
Article DOI: 10.1021/jm0611713
BindingDB Entry DOI: 10.7270/Q25H7H3J |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Mus musculus (Mouse)) | BDBM50076440

(9-[5-(8-Methyl-2-oxo-4H-2lambda*5*-benzo[1,3,2]dio...)Show SMILES Cc1cccc2COP(=O)(OCC3CCC(O3)n3cnc4c(N)ncnc34)Oc12 Show InChI InChI=1S/C18H20N5O5P/c1-11-3-2-4-12-7-25-29(24,28-16(11)12)26-8-13-5-6-14(27-13)23-10-22-15-17(19)20-9-21-18(15)23/h2-4,9-10,13-14H,5-8H2,1H3,(H2,19,20,21) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards mouse butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50146886
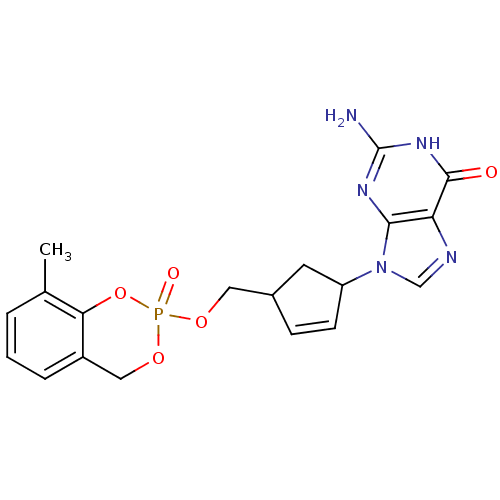
(2-Amino-9-[4-(8-methyl-2-oxo-4H-2lambda*5*-benzo[1...)Show SMILES Cc1cccc2COP(=O)(OCC3CC(C=C3)n3cnc4c3nc(N)[nH]c4=O)Oc12 |c:15| Show InChI InChI=1S/C19H20N5O5P/c1-11-3-2-4-13-9-28-30(26,29-16(11)13)27-8-12-5-6-14(7-12)24-10-21-15-17(24)22-19(20)23-18(15)25/h2-6,10,12,14H,7-9H2,1H3,(H3,20,22,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50422515

(CHEMBL2114301)Show SMILES Cc1cn([C@@H]2O[C@H](CO[P@]3(=O)OCc4cccc(c4O3)C(C)(C)C)C=C2)c(=O)[nH]c1=O |r,c:26| Show InChI InChI=1S/C21H25N2O7P/c1-13-10-23(20(25)22-19(13)24)17-9-8-15(29-17)12-28-31(26)27-11-14-6-5-7-16(18(14)30-31)21(2,3)4/h5-10,15,17H,11-12H2,1-4H3,(H,22,24,25)/t15-,17+,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50146873
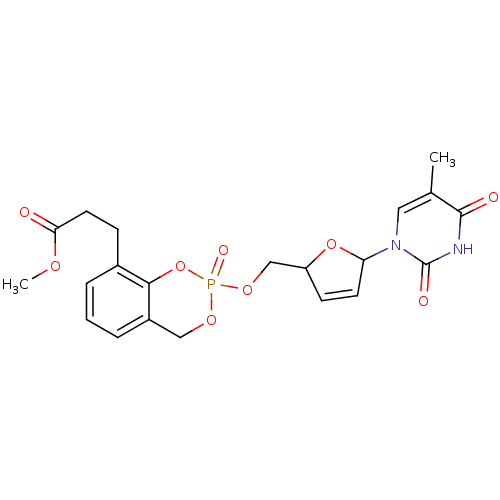
(3-{2-[5-(5-Methyl-2,4-dioxo-3,4-dihydro-2H-pyrimid...)Show SMILES COC(=O)CCc1cccc2COP(=O)(OCC3OC(C=C3)n3cc(C)c(=O)[nH]c3=O)Oc12 |c:20| Show InChI InChI=1S/C21H23N2O9P/c1-13-10-23(21(26)22-20(13)25)17-8-7-16(31-17)12-30-33(27)29-11-15-5-3-4-14(19(15)32-33)6-9-18(24)28-2/h3-5,7-8,10,16-17H,6,9,11-12H2,1-2H3,(H,22,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50063918

((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-1-methyl...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)[C@H](CSc1cccs1)C(=O)NO Show InChI InChI=1S/C23H31N3O4S2/c1-15(2)12-17(18(22(28)26-30)14-32-20-10-7-11-31-20)21(27)25-19(23(29)24-3)13-16-8-5-4-6-9-16/h4-11,15,17-19,30H,12-14H2,1-3H3,(H,24,29)(H,25,27)(H,26,28)/t17-,18+,19+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00187f
BindingDB Entry DOI: 10.7270/Q2N01BG9 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50146864
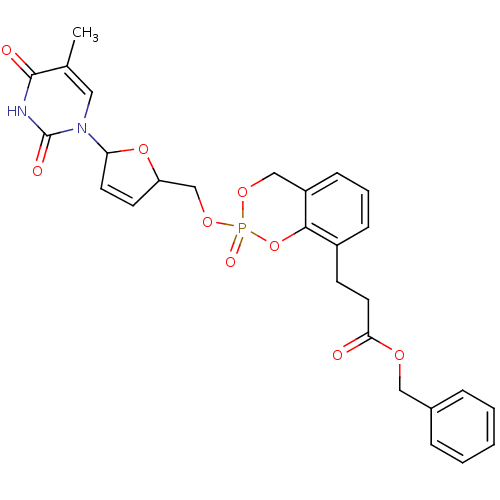
(3-{2-[5-(5-Methyl-2,4-dioxo-3,4-dihydro-2H-pyrimid...)Show SMILES Cc1cn(C2OC(COP3(=O)OCc4cccc(CCC(=O)OCc5ccccc5)c4O3)C=C2)c(=O)[nH]c1=O |c:35| Show InChI InChI=1S/C27H27N2O9P/c1-18-14-29(27(32)28-26(18)31)23-12-11-22(37-23)17-36-39(33)35-16-21-9-5-8-20(25(21)38-39)10-13-24(30)34-15-19-6-3-2-4-7-19/h2-9,11-12,14,22-23H,10,13,15-17H2,1H3,(H,28,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50146877

(1-[5-(6-sec-Butyl-2-oxo-4H-2lambda*5*-benzo[1,3,2]...)Show SMILES CCC(C)c1ccc2OP(=O)(OCC3OC(C=C3)n3cc(C)c(=O)[nH]c3=O)OCc2c1 |c:16| Show InChI InChI=1S/C21H25N2O7P/c1-4-13(2)15-5-7-18-16(9-15)11-27-31(26,30-18)28-12-17-6-8-19(29-17)23-10-14(3)20(24)22-21(23)25/h5-10,13,17,19H,4,11-12H2,1-3H3,(H,22,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50146876

(3-{2-[5-(5-Methyl-2,4-dioxo-3,4-dihydro-2H-pyrimid...)Show SMILES Cc1cn(C2OC(COP3(=O)OCc4cccc(CCC(=O)OC(C)(C)C)c4O3)C=C2)c(=O)[nH]c1=O |c:31| Show InChI InChI=1S/C24H29N2O9P/c1-15-12-26(23(29)25-22(15)28)19-10-9-18(33-19)14-32-36(30)31-13-17-7-5-6-16(21(17)35-36)8-11-20(27)34-24(2,3)4/h5-7,9-10,12,18-19H,8,11,13-14H2,1-4H3,(H,25,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
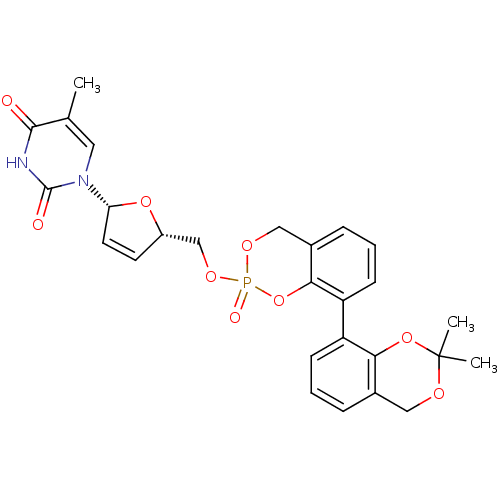
(Homo sapiens (Human)) | BDBM50206632
((Rp)-3-isopr-sal-cyclosal-d4TMP | (Sp)-3-isopr-sal...)Show SMILES Cc1cn([C@@H]2O[C@H](COP3(=O)OCc4cccc(c4O3)-c3cccc4COC(C)(C)Oc34)C=C2)c(=O)[nH]c1=O |c:36| Show InChI InChI=1S/C27H27N2O9P/c1-16-12-29(26(31)28-25(16)30)22-11-10-19(36-22)15-35-39(32)34-14-18-7-5-9-21(24(18)38-39)20-8-4-6-17-13-33-27(2,3)37-23(17)20/h4-12,19,22H,13-15H2,1-3H3,(H,28,30,31)/t19-,22+,39?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE |
J Med Chem 50: 1335-46 (2007)
Article DOI: 10.1021/jm0611713
BindingDB Entry DOI: 10.7270/Q25H7H3J |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50422516

(CHEMBL2114303)Show SMILES O[C@H]1C[C@@H](O[C@@H]1CO[P@]1(=O)OCc2cccc(-c3ccccc3)c2O1)N1CC(=CCBr)C(=O)NC1=O |r,w:28.32| Show InChI InChI=1S/C24H24BrN2O8P/c25-10-9-16-12-27(24(30)26-23(16)29)21-11-19(28)20(34-21)14-33-36(31)32-13-17-7-4-8-18(22(17)35-36)15-5-2-1-3-6-15/h1-9,19-21,28H,10-14H2,(H,26,29,30)/t19-,20+,21+,36-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50146889

(5-Methyl-1-[5-(2-oxo-4H-1,3-dioxa-2lambda*5*-phosp...)Show SMILES Cc1cn(C2OC(COP3(=O)OCc4c(O3)ccc3ccccc43)C=C2)c(=O)[nH]c1=O |c:27| Show InChI InChI=1S/C21H19N2O7P/c1-13-10-23(21(25)22-20(13)24)19-9-7-15(29-19)11-27-31(26)28-12-17-16-5-3-2-4-14(16)6-8-18(17)30-31/h2-10,15,19H,11-12H2,1H3,(H,22,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50146851
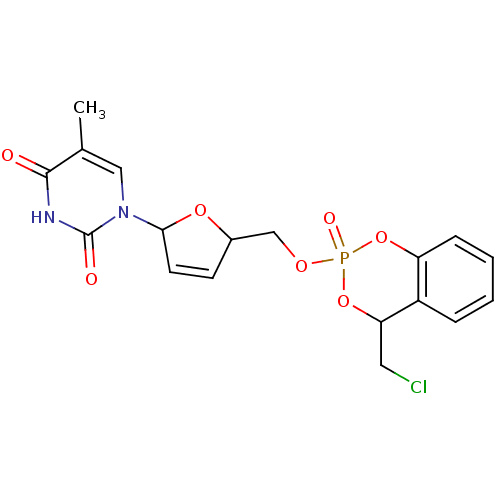
(1-[5-(4-Chloromethyl-2-oxo-4H-2lambda*5*-benzo[1,3...)Show SMILES Cc1cn(C2OC(COP3(=O)OC(CCl)c4ccccc4O3)C=C2)c(=O)[nH]c1=O |c:24| Show InChI InChI=1S/C18H18ClN2O7P/c1-11-9-21(18(23)20-17(11)22)16-7-6-12(26-16)10-25-29(24)27-14-5-3-2-4-13(14)15(8-19)28-29/h2-7,9,12,15-16H,8,10H2,1H3,(H,20,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50422507

(CHEMBL375567)Show SMILES Cc1cn([C@@H]2O[C@H](COP3(=O)OCc4cccc(c4O3)C(C)(C)C)C=C2)c(=O)[nH]c1=O |c:26| Show InChI InChI=1S/C21H25N2O7P/c1-13-10-23(20(25)22-19(13)24)17-9-8-15(29-17)12-28-31(26)27-11-14-6-5-7-16(18(14)30-31)21(2,3)4/h5-10,15,17H,11-12H2,1-4H3,(H,22,24,25)/t15-,17+,31?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50146856
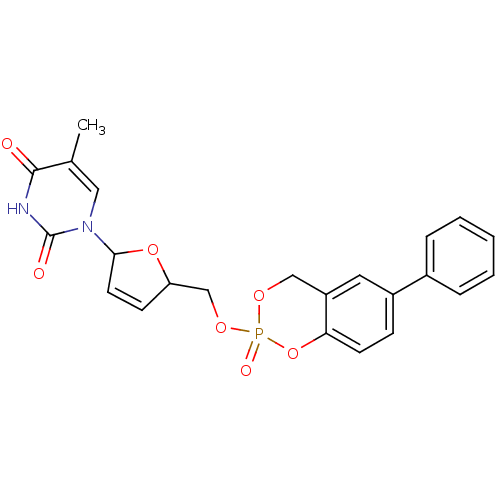
(5-Methyl-1-[5-(2-oxo-6-phenyl-4H-2lambda*5*-benzo[...)Show SMILES Cc1cn(C2OC(COP3(=O)OCc4cc(ccc4O3)-c3ccccc3)C=C2)c(=O)[nH]c1=O |c:29| Show InChI InChI=1S/C23H21N2O7P/c1-15-12-25(23(27)24-22(15)26)21-10-8-19(31-21)14-30-33(28)29-13-18-11-17(7-9-20(18)32-33)16-5-3-2-4-6-16/h2-12,19,21H,13-14H2,1H3,(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50146853

(3-{2-[5-(5-Methyl-2,4-dioxo-3,4-dihydro-2H-pyrimid...)Show SMILES Cc1cn(C2OC(COP3(=O)OCc4cc(CCC(=O)OC(C)(C)C)ccc4O3)C=C2)c(=O)[nH]c1=O |c:31| Show InChI InChI=1S/C24H29N2O9P/c1-15-12-26(23(29)25-22(15)28)20-9-7-18(33-20)14-32-36(30)31-13-17-11-16(5-8-19(17)35-36)6-10-21(27)34-24(2,3)4/h5,7-9,11-12,18,20H,6,10,13-14H2,1-4H3,(H,25,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data