

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 1/REST corepressor 3 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of HDAC1/CoREST3 in HEK293 whole cell extract using fluorescent acetylated histone peptide as substrate after 60 mins by fluorescence base... | Bioorg Med Chem Lett 28: 1001-1004 (2018) Article DOI: 10.1016/j.bmcl.2018.02.034 BindingDB Entry DOI: 10.7270/Q2280B78 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM15581 (CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Irreversible inhibition of recombinant human MAO-A expressed in baculovirus infected BT1 cells using p-tyramine as substrate by Lineweaver-Burk plot ... | Eur J Med Chem 70: 88-101 (2013) Article DOI: 10.1016/j.ejmech.2013.07.005 BindingDB Entry DOI: 10.7270/Q2T156MV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 1/REST corepressor 3 (Homo sapiens (Human)) | BDBM50460385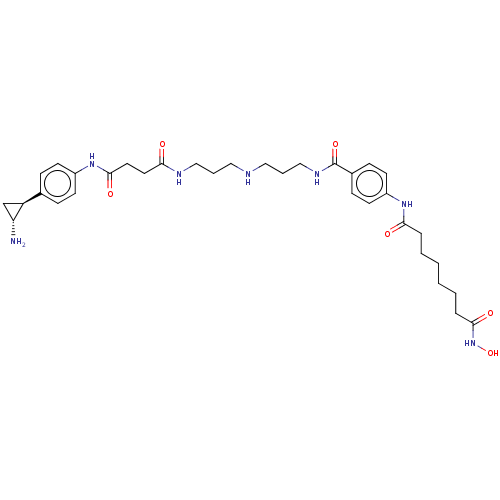 (CHEMBL4228572) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of HDAC1/CoREST3 in HEK293 whole cell extract using fluorescent acetylated histone peptide as substrate after 60 mins by fluorescence base... | Bioorg Med Chem Lett 28: 1001-1004 (2018) Article DOI: 10.1016/j.bmcl.2018.02.034 BindingDB Entry DOI: 10.7270/Q2280B78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1/REST corepressor 3 (Homo sapiens (Human)) | BDBM50460386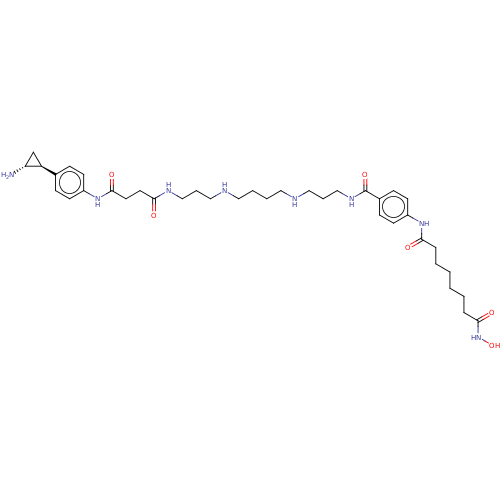 (CHEMBL4228166) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of HDAC1/CoREST3 in HEK293 whole cell extract using fluorescent acetylated histone peptide as substrate after 60 mins by fluorescence base... | Bioorg Med Chem Lett 28: 1001-1004 (2018) Article DOI: 10.1016/j.bmcl.2018.02.034 BindingDB Entry DOI: 10.7270/Q2280B78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM15579 (CHEMBL972 | DEPRENYL | L-Deprenyl | N-methyl-N-[(2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Irreversible inhibition of recombinant human MAO-B expressed in baculovirus infected BT1 cells using benzylamine as substrate at 200 uM preincubated ... | Eur J Med Chem 70: 88-101 (2013) Article DOI: 10.1016/j.ejmech.2013.07.005 BindingDB Entry DOI: 10.7270/Q2T156MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM11022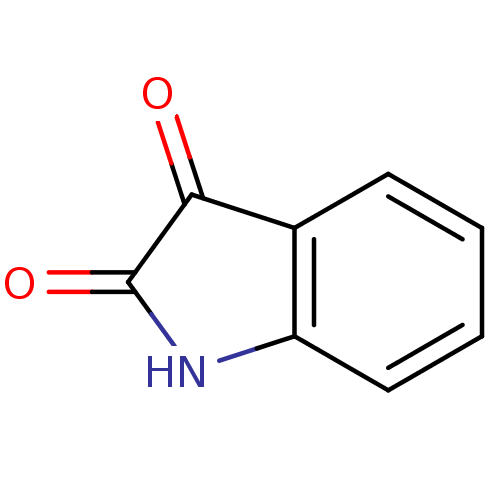 (2,3-dihydro-1H-indole-2,3-dione | CHEMBL326294 | I...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Competitive inhibition of recombinant human MAO-B expressed in baculovirus infected BT1 cells using benzylamine as substrate at 200 uM preincubated f... | Eur J Med Chem 70: 88-101 (2013) Article DOI: 10.1016/j.ejmech.2013.07.005 BindingDB Entry DOI: 10.7270/Q2T156MV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50494371 (CHEMBL3086339) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Competitive inhibition of MAOA in human SH-SY5Y cells using p-tyramine as substrate preincubated for 5 mins by Lineweaver-Burk plot analysis | Eur J Med Chem 70: 88-101 (2013) Article DOI: 10.1016/j.ejmech.2013.07.005 BindingDB Entry DOI: 10.7270/Q2T156MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50494371 (CHEMBL3086339) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Irreversible inhibition of recombinant human MAO-A expressed in baculovirus infected BT1 cells using p-tyramine as substrate by Lineweaver-Burk plot ... | Eur J Med Chem 70: 88-101 (2013) Article DOI: 10.1016/j.ejmech.2013.07.005 BindingDB Entry DOI: 10.7270/Q2T156MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50494371 (CHEMBL3086339) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Competitive inhibition of recombinant human MAO-B expressed in baculovirus infected BT1 cells using benzylamine as substrate at 200 uM preincubated f... | Eur J Med Chem 70: 88-101 (2013) Article DOI: 10.1016/j.ejmech.2013.07.005 BindingDB Entry DOI: 10.7270/Q2T156MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM11022 (2,3-dihydro-1H-indole-2,3-dione | CHEMBL326294 | I...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Competitive inhibition of recombinant human MAO-A expressed in baculovirus infected BT1 cells using p-tyramine as substrate by Lineweaver-Burk plot a... | Eur J Med Chem 70: 88-101 (2013) Article DOI: 10.1016/j.ejmech.2013.07.005 BindingDB Entry DOI: 10.7270/Q2T156MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50494369 (CHEMBL3086344) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Mixed type inhibition of recombinant human MAO-B expressed in baculovirus infected BT1 cells using benzylamine as substrate at 200 uM preincubated fo... | Eur J Med Chem 70: 88-101 (2013) Article DOI: 10.1016/j.ejmech.2013.07.005 BindingDB Entry DOI: 10.7270/Q2T156MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50494370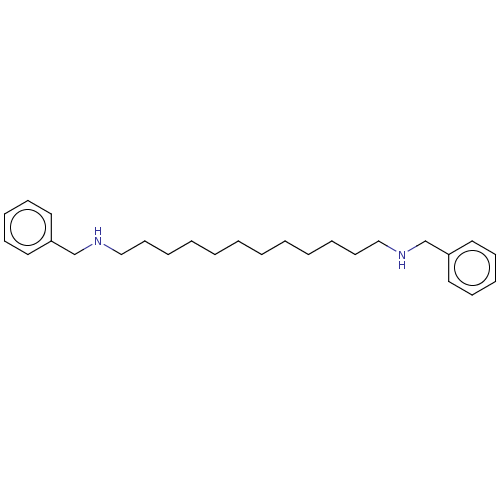 (CHEMBL3086341) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Mixed type inhibition of recombinant human MAO-A expressed in baculovirus infected BT1 cells using p-tyramine as substrate by Lineweaver-Burk plot an... | Eur J Med Chem 70: 88-101 (2013) Article DOI: 10.1016/j.ejmech.2013.07.005 BindingDB Entry DOI: 10.7270/Q2T156MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50494370 (CHEMBL3086341) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.29E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Mixed type inhibition of recombinant human MAO-B expressed in baculovirus infected BT1 cells using benzylamine as substrate at 200 uM preincubated fo... | Eur J Med Chem 70: 88-101 (2013) Article DOI: 10.1016/j.ejmech.2013.07.005 BindingDB Entry DOI: 10.7270/Q2T156MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50494368 (CHEMBL1185605) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Mixed type inhibition of recombinant human MAO-A expressed in baculovirus infected BT1 cells using p-tyramine as substrate by Lineweaver-Burk plot an... | Eur J Med Chem 70: 88-101 (2013) Article DOI: 10.1016/j.ejmech.2013.07.005 BindingDB Entry DOI: 10.7270/Q2T156MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50494370 (CHEMBL3086341) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.86E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Reversible inhibition of recombinant human MAO-A expressed in baculovirus infected BT1 cells using p-tyramine as substrate by Lineweaver-Burk plot an... | Eur J Med Chem 70: 88-101 (2013) Article DOI: 10.1016/j.ejmech.2013.07.005 BindingDB Entry DOI: 10.7270/Q2T156MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50494368 (CHEMBL1185605) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.88E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Reversible inhibition of recombinant human MAO-A expressed in baculovirus infected BT1 cells using p-tyramine as substrate by Lineweaver-Burk plot an... | Eur J Med Chem 70: 88-101 (2013) Article DOI: 10.1016/j.ejmech.2013.07.005 BindingDB Entry DOI: 10.7270/Q2T156MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50494369 (CHEMBL3086344) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.26E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Mixed type inhibition of recombinant human MAO-A expressed in baculovirus infected BT1 cells using p-tyramine as substrate by Lineweaver-Burk plot an... | Eur J Med Chem 70: 88-101 (2013) Article DOI: 10.1016/j.ejmech.2013.07.005 BindingDB Entry DOI: 10.7270/Q2T156MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50494368 (CHEMBL1185605) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.91E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Mixed type inhibition of recombinant human MAO-B expressed in baculovirus infected BT1 cells using benzylamine as substrate at 200 uM preincubated fo... | Eur J Med Chem 70: 88-101 (2013) Article DOI: 10.1016/j.ejmech.2013.07.005 BindingDB Entry DOI: 10.7270/Q2T156MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50494369 (CHEMBL3086344) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.14E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Reversible inhibition of recombinant human MAO-B expressed in baculovirus infected BT1 cells using benzylamine as substrate at 200 uM preincubated fo... | Eur J Med Chem 70: 88-101 (2013) Article DOI: 10.1016/j.ejmech.2013.07.005 BindingDB Entry DOI: 10.7270/Q2T156MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50494368 (CHEMBL1185605) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.85E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Reversible inhibition of recombinant human MAO-B expressed in baculovirus infected BT1 cells using benzylamine as substrate at 200 uM preincubated fo... | Eur J Med Chem 70: 88-101 (2013) Article DOI: 10.1016/j.ejmech.2013.07.005 BindingDB Entry DOI: 10.7270/Q2T156MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50494369 (CHEMBL3086344) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.97E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Reversible inhibition of recombinant human MAO-A expressed in baculovirus infected BT1 cells using p-tyramine as substrate by Lineweaver-Burk plot an... | Eur J Med Chem 70: 88-101 (2013) Article DOI: 10.1016/j.ejmech.2013.07.005 BindingDB Entry DOI: 10.7270/Q2T156MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50494370 (CHEMBL3086341) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.05E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Reversible inhibition of recombinant human MAO-B expressed in baculovirus infected BT1 cells using benzylamine as substrate at 200 uM preincubated fo... | Eur J Med Chem 70: 88-101 (2013) Article DOI: 10.1016/j.ejmech.2013.07.005 BindingDB Entry DOI: 10.7270/Q2T156MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM13061 (4,4 -(1H-1,2,4-triazol-1-ylmethanediyl)dibenzonitr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human aromatase assessed as reduction in fluorescence intensity preincubated with NADPH regenerating system for 10 mins followed by sub... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113733 BindingDB Entry DOI: 10.7270/Q2F76HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50582046 (CHEMBL5080066) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Induction of degradation of ERalpha in human MCF7 cells at 72 hrs by Western blot analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113733 BindingDB Entry DOI: 10.7270/Q2F76HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50582046 (CHEMBL5080066) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human ERalpha measured after 2 hrs by fluorescence polarization plate reader | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113733 BindingDB Entry DOI: 10.7270/Q2F76HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50582046 (CHEMBL5080066) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human aromatase assessed as reduction in fluorescence intensity preincubated with NADPH regenerating system for 10 mins followed by sub... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113733 BindingDB Entry DOI: 10.7270/Q2F76HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50435003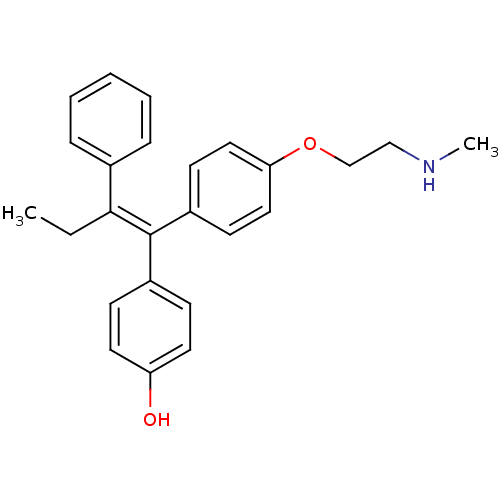 (ENDOXIFEN) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human ERalpha measured after 2 hrs by fluorescence polarization plate reader | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113733 BindingDB Entry DOI: 10.7270/Q2F76HFC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50582050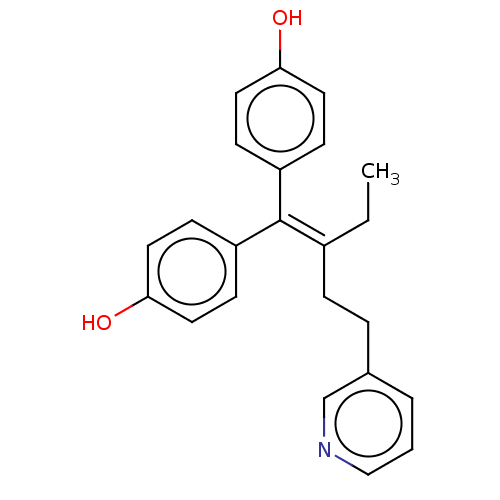 (CHEMBL5092560) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Induction of degradation of ERalpha in human MCF7 cells at 72 hrs by Western blot analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113733 BindingDB Entry DOI: 10.7270/Q2F76HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50582050 (CHEMBL5092560) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human ERalpha measured after 2 hrs by fluorescence polarization plate reader | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113733 BindingDB Entry DOI: 10.7270/Q2F76HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50582048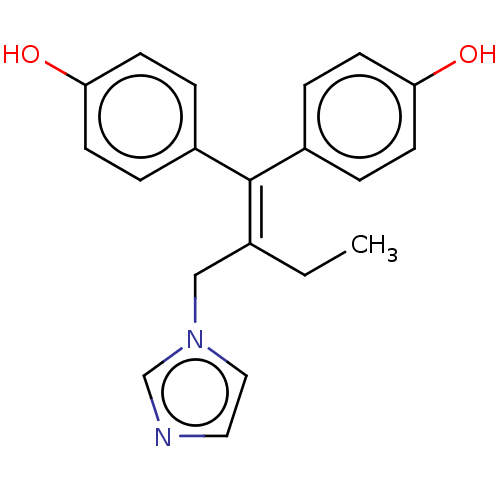 (CHEMBL5072101) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human aromatase assessed as reduction in fluorescence intensity preincubated with NADPH regenerating system for 10 mins followed by sub... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113733 BindingDB Entry DOI: 10.7270/Q2F76HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50582052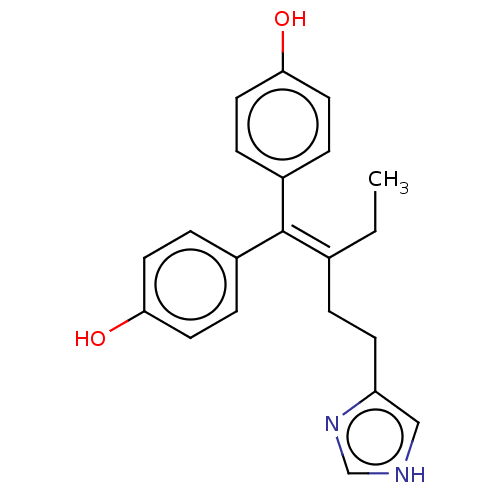 (CHEMBL5074047) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human aromatase assessed as reduction in fluorescence intensity preincubated with NADPH regenerating system for 10 mins followed by sub... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113733 BindingDB Entry DOI: 10.7270/Q2F76HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50582053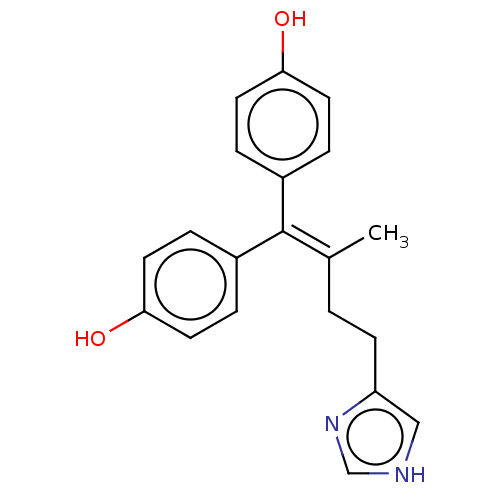 (CHEMBL5081446) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human aromatase assessed as reduction in fluorescence intensity preincubated with NADPH regenerating system for 10 mins followed by sub... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113733 BindingDB Entry DOI: 10.7270/Q2F76HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50582043 (CHEMBL5077976) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human ERalpha measured after 2 hrs by fluorescence polarization plate reader | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113733 BindingDB Entry DOI: 10.7270/Q2F76HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50582052 (CHEMBL5074047) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 197 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human ERalpha measured after 2 hrs by fluorescence polarization plate reader | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113733 BindingDB Entry DOI: 10.7270/Q2F76HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50582045 (CHEMBL5082681) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 223 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Induction of degradation of ERalpha in human MCF7 cells at 72 hrs by Western blot analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113733 BindingDB Entry DOI: 10.7270/Q2F76HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50582045 (CHEMBL5082681) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 223 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human ERalpha measured after 2 hrs by fluorescence polarization plate reader | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113733 BindingDB Entry DOI: 10.7270/Q2F76HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50582051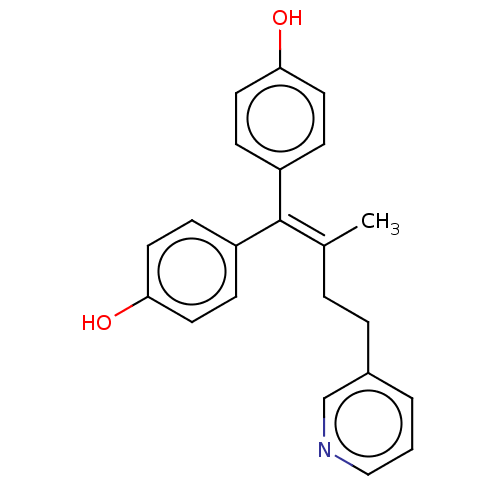 (CHEMBL5092843) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 313 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human ERalpha measured after 2 hrs by fluorescence polarization plate reader | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113733 BindingDB Entry DOI: 10.7270/Q2F76HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50582045 (CHEMBL5082681) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 596 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human aromatase assessed as reduction in fluorescence intensity preincubated with NADPH regenerating system for 10 mins followed by sub... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113733 BindingDB Entry DOI: 10.7270/Q2F76HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50582047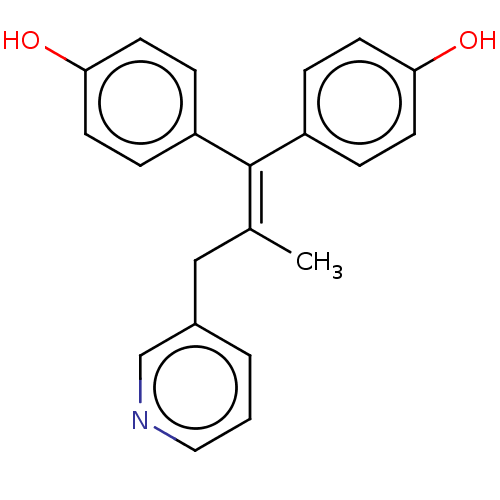 (CHEMBL5069811) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 861 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human aromatase assessed as reduction in fluorescence intensity preincubated with NADPH regenerating system for 10 mins followed by sub... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113733 BindingDB Entry DOI: 10.7270/Q2F76HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50582050 (CHEMBL5092560) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human aromatase assessed as reduction in fluorescence intensity preincubated with NADPH regenerating system for 10 mins followed by sub... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113733 BindingDB Entry DOI: 10.7270/Q2F76HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50582044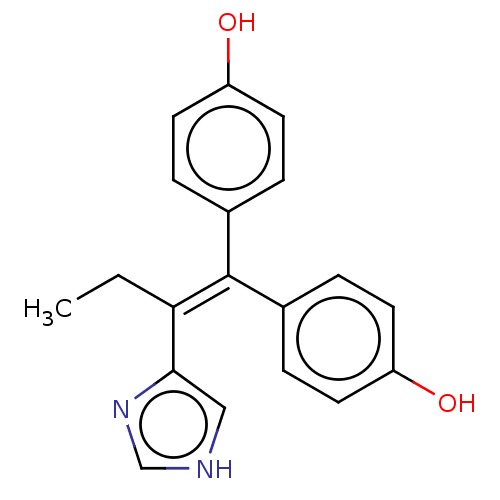 (CHEMBL5075144) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 952 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human aromatase assessed as reduction in fluorescence intensity preincubated with NADPH regenerating system for 10 mins followed by sub... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113733 BindingDB Entry DOI: 10.7270/Q2F76HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50582049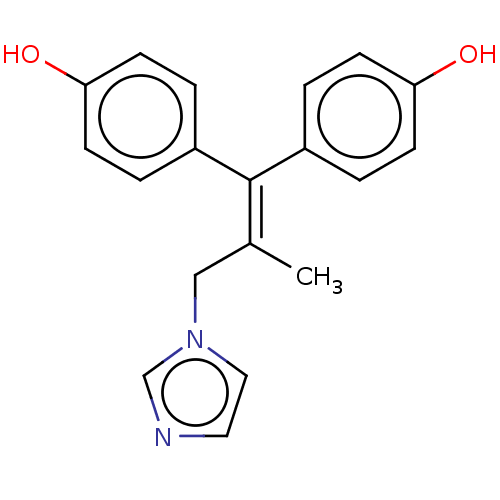 (CHEMBL5087363) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human aromatase assessed as reduction in fluorescence intensity preincubated with NADPH regenerating system for 10 mins followed by sub... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113733 BindingDB Entry DOI: 10.7270/Q2F76HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50582044 (CHEMBL5075144) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human ERalpha measured after 2 hrs by fluorescence polarization plate reader | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113733 BindingDB Entry DOI: 10.7270/Q2F76HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50582042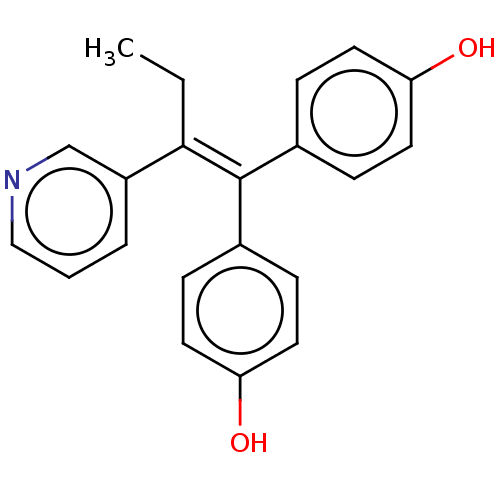 (CHEMBL5082245) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human aromatase assessed as reduction in fluorescence intensity preincubated with NADPH regenerating system for 10 mins followed by sub... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113733 BindingDB Entry DOI: 10.7270/Q2F76HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM50501616 (CHEMBL4084089) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of human topoisomerase-2alpha-mediated relaxation of supercoiled pBR322 DNA after 1 hr by ethidium bromide staining based agarose gel elec... | Eur J Med Chem 128: 107-122 (2017) Article DOI: 10.1016/j.ejmech.2017.01.025 BindingDB Entry DOI: 10.7270/Q2668H7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50582053 (CHEMBL5081446) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human ERalpha measured after 2 hrs by fluorescence polarization plate reader | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113733 BindingDB Entry DOI: 10.7270/Q2F76HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50582047 (CHEMBL5069811) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human ERalpha measured after 2 hrs by fluorescence polarization plate reader | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113733 BindingDB Entry DOI: 10.7270/Q2F76HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50582048 (CHEMBL5072101) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Induction of degradation of ERalpha in human MCF7 cells at 72 hrs by Western blot analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113733 BindingDB Entry DOI: 10.7270/Q2F76HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50582048 (CHEMBL5072101) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human ERalpha measured after 2 hrs by fluorescence polarization plate reader | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113733 BindingDB Entry DOI: 10.7270/Q2F76HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM50501615 (CHEMBL4091846) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of human topoisomerase-2alpha-mediated relaxation of supercoiled pBR322 DNA after 1 hr by ethidium bromide staining based agarose gel elec... | Eur J Med Chem 128: 107-122 (2017) Article DOI: 10.1016/j.ejmech.2017.01.025 BindingDB Entry DOI: 10.7270/Q2668H7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 67 total ) | Next | Last >> |