

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM395298 (6-(Difluoromethyl)-N-[2-(3-hydroxy-3-methylbutyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM DMSO solution of the test substance. 50... | US Patent US10793545 (2020) BindingDB Entry DOI: 10.7270/Q29S1V3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM395298 (6-(Difluoromethyl)-N-[2-(3-hydroxy-3-methylbutyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen | Assay Description For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM DMSO solution of the test substance. 50... | Bioorg Med Chem Lett 19: 3550-4 (2009) BindingDB Entry DOI: 10.7270/Q2J67K7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM395296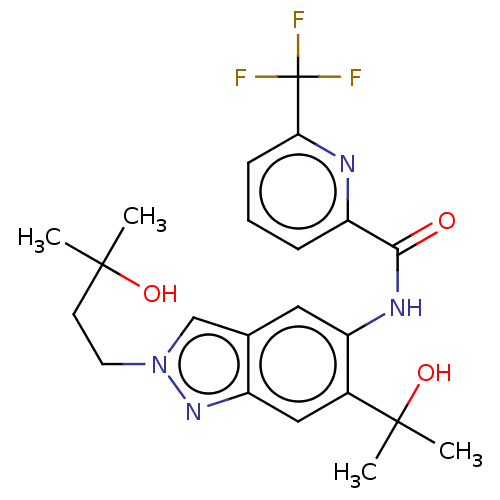 (N-[2-(3-Hydroxy-3-methylbutyl)-6-(2-hydroxypropan-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM DMSO solution of the test substance. 50... | US Patent US10793545 (2020) BindingDB Entry DOI: 10.7270/Q29S1V3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM395296 (N-[2-(3-Hydroxy-3-methylbutyl)-6-(2-hydroxypropan-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen | Assay Description For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM DMSO solution of the test substance. 50... | Bioorg Med Chem Lett 19: 3550-4 (2009) BindingDB Entry DOI: 10.7270/Q2J67K7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM395307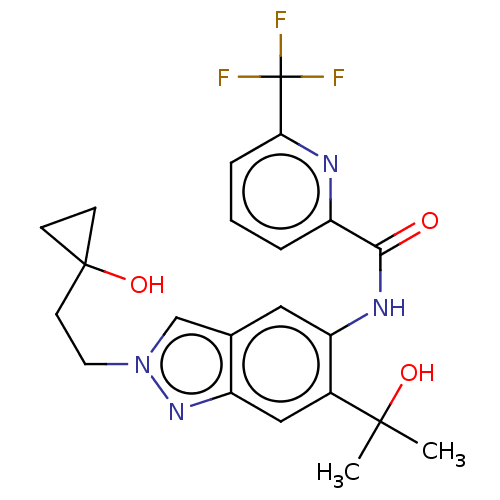 (N-{2-[2-(1-Hydroxycyclopropyl)ethyl]-6-(2-hydroxyp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM DMSO solution of the test substance. 50... | US Patent US10793545 (2020) BindingDB Entry DOI: 10.7270/Q29S1V3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM395307 (N-{2-[2-(1-Hydroxycyclopropyl)ethyl]-6-(2-hydroxyp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen | Assay Description For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM DMSO solution of the test substance. 50... | Bioorg Med Chem Lett 19: 3550-4 (2009) BindingDB Entry DOI: 10.7270/Q2J67K7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM395288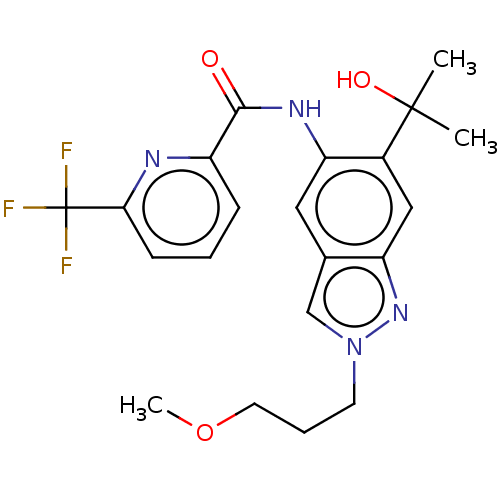 (N-[6-(2-Hydroxypropan-2-yl)-2-(3-methoxypropyl)-2H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM DMSO solution of the test substance. 50... | US Patent US10793545 (2020) BindingDB Entry DOI: 10.7270/Q29S1V3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM395288 (N-[6-(2-Hydroxypropan-2-yl)-2-(3-methoxypropyl)-2H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen | Assay Description For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM DMSO solution of the test substance. 50... | Bioorg Med Chem Lett 19: 3550-4 (2009) BindingDB Entry DOI: 10.7270/Q2J67K7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM395304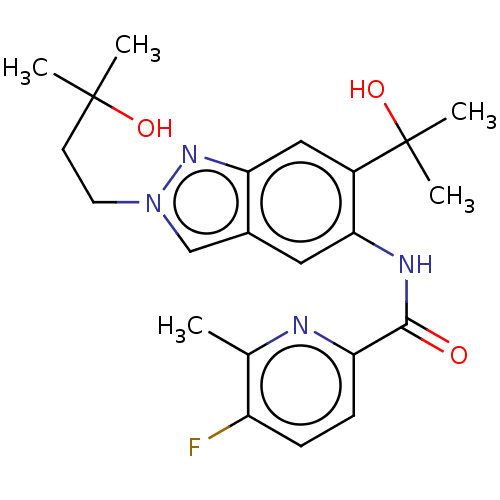 (5-Fluoro-N-[2-(3-hydroxy-3-methylbutyl)-6-(2-hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen | Assay Description For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM DMSO solution of the test substance. 50... | Bioorg Med Chem Lett 19: 3550-4 (2009) BindingDB Entry DOI: 10.7270/Q2J67K7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM395304 (5-Fluoro-N-[2-(3-hydroxy-3-methylbutyl)-6-(2-hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM DMSO solution of the test substance. 50... | US Patent US10793545 (2020) BindingDB Entry DOI: 10.7270/Q29S1V3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50548486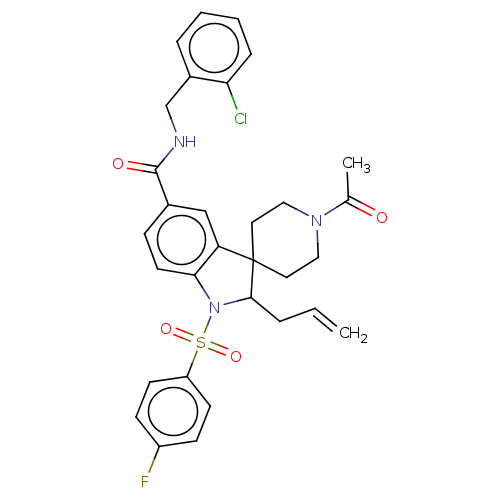 (CHEMBL4741994) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human GnRH receptor expressed in CHO-K1 cells assessed as inhibition of LHRH-induced response preincubated for 20 mins followe... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01076 BindingDB Entry DOI: 10.7270/Q26D5XK9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50548475 (CHEMBL4747248) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human GnRH receptor expressed in CHO-K1 cells assessed as inhibition of LHRH-induced response preincubated for 20 mins followe... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01076 BindingDB Entry DOI: 10.7270/Q26D5XK9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM395297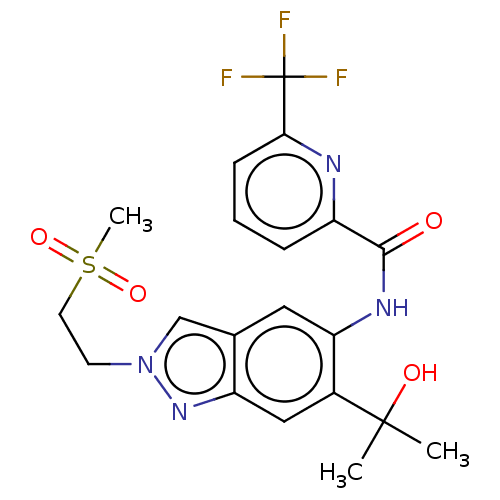 (N-{6-(2-Hydroxypropan-2-yl)-2-[2-(methylsulphonyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 10.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM DMSO solution of the test substance. 50... | US Patent US10793545 (2020) BindingDB Entry DOI: 10.7270/Q29S1V3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM395297 (N-{6-(2-Hydroxypropan-2-yl)-2-[2-(methylsulphonyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 10.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen | Assay Description For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM DMSO solution of the test substance. 50... | Bioorg Med Chem Lett 19: 3550-4 (2009) BindingDB Entry DOI: 10.7270/Q2J67K7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM395299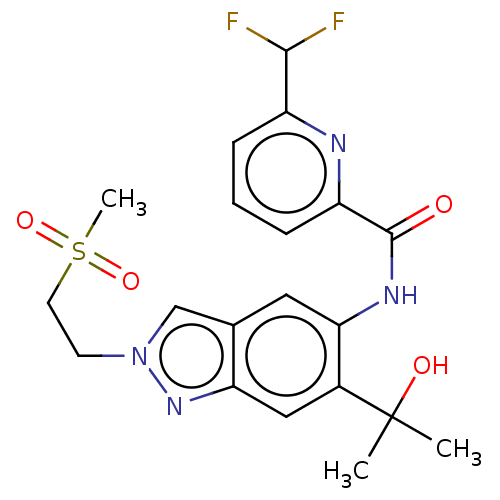 (6-(Difluoromethyl)-N-{6-(2-hydroxypropan-2-yl)-2-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen | Assay Description For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM DMSO solution of the test substance. 50... | Bioorg Med Chem Lett 19: 3550-4 (2009) BindingDB Entry DOI: 10.7270/Q2J67K7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM395299 (6-(Difluoromethyl)-N-{6-(2-hydroxypropan-2-yl)-2-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM DMSO solution of the test substance. 50... | US Patent US10793545 (2020) BindingDB Entry DOI: 10.7270/Q29S1V3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM395300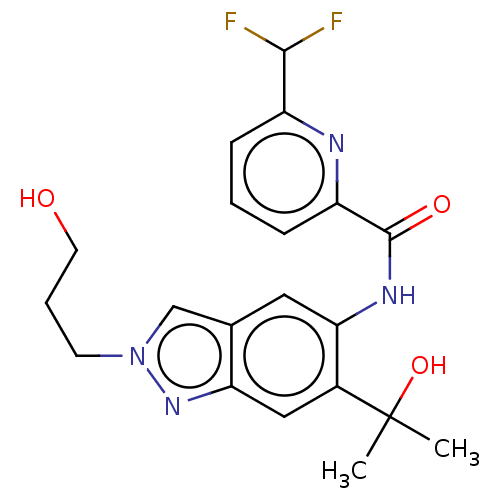 (6-(Difluoromethyl)-N-[6-(2-hydroxypropan-2-yl)-2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen | Assay Description For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM DMSO solution of the test substance. 50... | Bioorg Med Chem Lett 19: 3550-4 (2009) BindingDB Entry DOI: 10.7270/Q2J67K7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM395300 (6-(Difluoromethyl)-N-[6-(2-hydroxypropan-2-yl)-2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM DMSO solution of the test substance. 50... | US Patent US10793545 (2020) BindingDB Entry DOI: 10.7270/Q29S1V3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50548470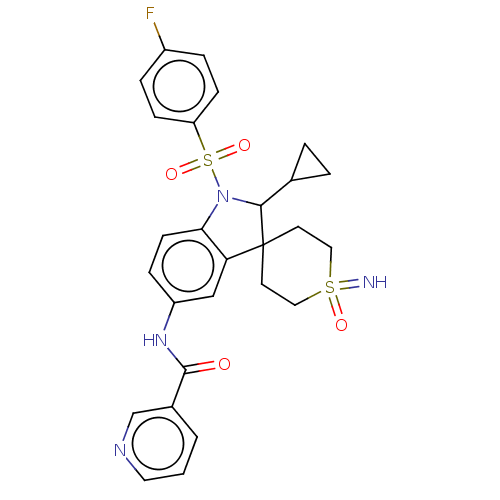 (CHEMBL4749974) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human GnRH receptor expressed in CHO-K1 cells assessed as inhibition of LHRH-induced response preincubated for 20 mins followe... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01076 BindingDB Entry DOI: 10.7270/Q26D5XK9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50548470 (CHEMBL4749974) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human GnRH receptor expressed in CHO-K1 cells assessed as inhibition of LHRH-induced response preincubated for 20 mins followe... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01076 BindingDB Entry DOI: 10.7270/Q26D5XK9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM395305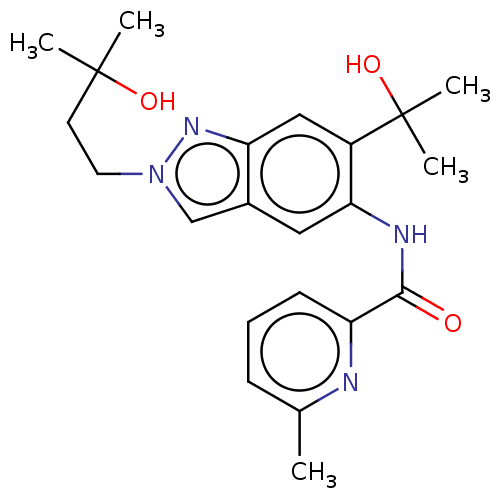 (N-[2-(3-Hydroxy-3-methylbutyl)-6-(2-hydroxypropan-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM DMSO solution of the test substance. 50... | US Patent US10793545 (2020) BindingDB Entry DOI: 10.7270/Q29S1V3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM395305 (N-[2-(3-Hydroxy-3-methylbutyl)-6-(2-hydroxypropan-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen | Assay Description For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM DMSO solution of the test substance. 50... | Bioorg Med Chem Lett 19: 3550-4 (2009) BindingDB Entry DOI: 10.7270/Q2J67K7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50548472 (CHEMBL4749790) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human GnRH receptor expressed in CHO-K1 cells assessed as inhibition of LHRH-induced response preincubated for 20 mins followe... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01076 BindingDB Entry DOI: 10.7270/Q26D5XK9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50548472 (CHEMBL4749790) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human GnRH receptor expressed in CHO-K1 cells assessed as inhibition of LHRH-induced response preincubated for 20 mins followe... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01076 BindingDB Entry DOI: 10.7270/Q26D5XK9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM395293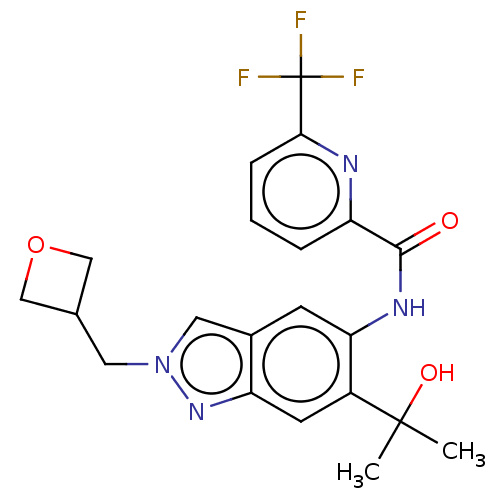 (N-[6-(2-Hydroxypropan-2-yl)-2-(oxetan-3-ylmethyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen | Assay Description For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM DMSO solution of the test substance. 50... | Bioorg Med Chem Lett 19: 3550-4 (2009) BindingDB Entry DOI: 10.7270/Q2J67K7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM395293 (N-[6-(2-Hydroxypropan-2-yl)-2-(oxetan-3-ylmethyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM DMSO solution of the test substance. 50... | US Patent US10793545 (2020) BindingDB Entry DOI: 10.7270/Q29S1V3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50041550 (CHEMBL3358413) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human GnRH receptor expressed in CHO-K1 cells assessed as inhibition of LHRH-induced response preincubated for 20 mins followe... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01076 BindingDB Entry DOI: 10.7270/Q26D5XK9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50548475 (CHEMBL4747248) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human GnRH receptor expressed in CHO-K1 cells assessed as inhibition of buserelin-induced response preincubated for 20 mins fo... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01076 BindingDB Entry DOI: 10.7270/Q26D5XK9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50548469 (CHEMBL4762368) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human GnRH receptor expressed in CHO-K1 cells assessed as inhibition of LHRH-induced response preincubated for 20 mins followe... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01076 BindingDB Entry DOI: 10.7270/Q26D5XK9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM395295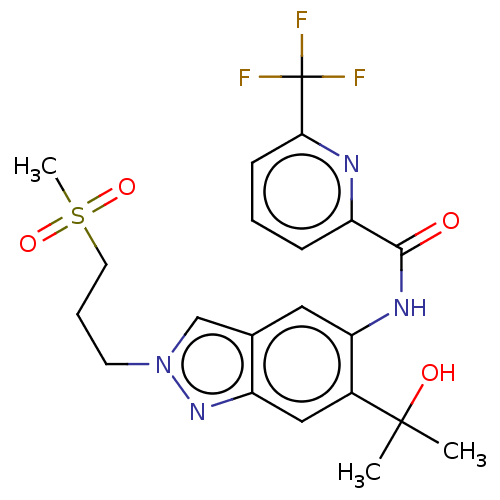 (N-{6-(2-Hydroxypropan-2-yl)-2-[3-(methylsulphonyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 18.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM DMSO solution of the test substance. 50... | US Patent US10793545 (2020) BindingDB Entry DOI: 10.7270/Q29S1V3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM395295 (N-{6-(2-Hydroxypropan-2-yl)-2-[3-(methylsulphonyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 18.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen | Assay Description For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM DMSO solution of the test substance. 50... | Bioorg Med Chem Lett 19: 3550-4 (2009) BindingDB Entry DOI: 10.7270/Q2J67K7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50548468 (CHEMBL4747322) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human GnRH receptor expressed in CHO-K1 cells assessed as inhibition of LHRH-induced response preincubated for 20 mins followe... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01076 BindingDB Entry DOI: 10.7270/Q26D5XK9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50548463 (CHEMBL4781434) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human GnRH receptor expressed in CHO-K1 cells assessed as inhibition of LHRH-induced response preincubated for 20 mins followe... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01076 BindingDB Entry DOI: 10.7270/Q26D5XK9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50548488 (CHEMBL4780737) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human GnRH receptor expressed in CHO-K1 cells assessed as inhibition of LHRH-induced response preincubated for 20 mins followe... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01076 BindingDB Entry DOI: 10.7270/Q26D5XK9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50548488 (CHEMBL4780737) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human GnRH receptor expressed in CHO-K1 cells assessed as inhibition of LHRH-induced response preincubated for 20 mins followe... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01076 BindingDB Entry DOI: 10.7270/Q26D5XK9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50548493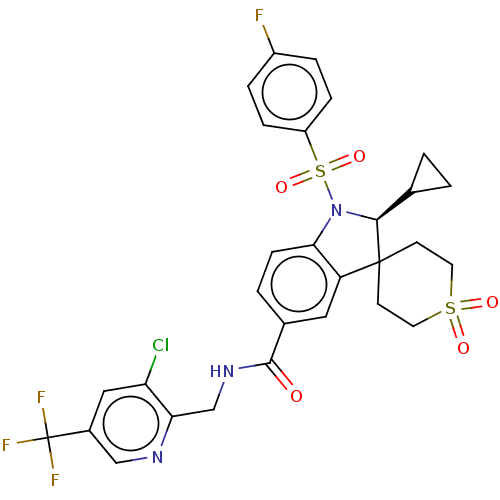 (BAY-784) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human GnRH receptor expressed in CHO-K1 cells assessed as inhibition of buserelin-induced response preincubated for 20 mins fo... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01076 BindingDB Entry DOI: 10.7270/Q26D5XK9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM395301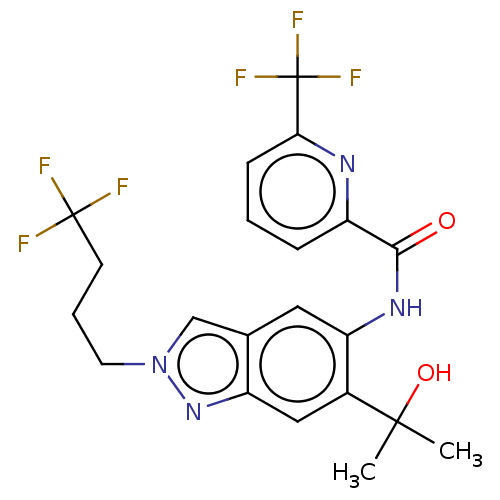 (N-[6-(2-Hydroxypropan-2-yl)-2-(4,4,4-trifluorobuty...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 21.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM DMSO solution of the test substance. 50... | US Patent US10793545 (2020) BindingDB Entry DOI: 10.7270/Q29S1V3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM395301 (N-[6-(2-Hydroxypropan-2-yl)-2-(4,4,4-trifluorobuty...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 21.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen | Assay Description For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM DMSO solution of the test substance. 50... | Bioorg Med Chem Lett 19: 3550-4 (2009) BindingDB Entry DOI: 10.7270/Q2J67K7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50548486 (CHEMBL4741994) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human GnRH receptor expressed in CHO-K1 cells assessed as inhibition of buserelin-induced response preincubated for 20 mins fo... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01076 BindingDB Entry DOI: 10.7270/Q26D5XK9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50548483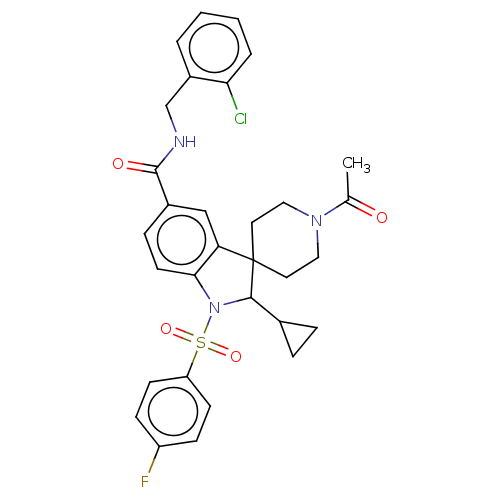 (CHEMBL4761024) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human GnRH receptor expressed in CHO-K1 cells assessed as inhibition of LHRH-induced response preincubated for 20 mins followe... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01076 BindingDB Entry DOI: 10.7270/Q26D5XK9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50548493 (BAY-784) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at rat GnRH receptor expressed in CHO-K1 cells assessed as inhibition of buserelin-induced response preincubated for 20 mins foll... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01076 BindingDB Entry DOI: 10.7270/Q26D5XK9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50548467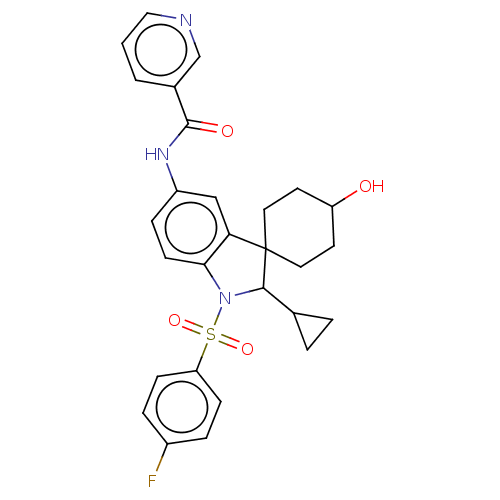 (CHEMBL4787856) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human GnRH receptor expressed in CHO-K1 cells assessed as inhibition of LHRH-induced response preincubated for 20 mins followe... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01076 BindingDB Entry DOI: 10.7270/Q26D5XK9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50548470 (CHEMBL4749974) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human GnRH receptor expressed in CHO-K1 cells assessed as inhibition of buserelin-induced response preincubated for 20 mins fo... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01076 BindingDB Entry DOI: 10.7270/Q26D5XK9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50548470 (CHEMBL4749974) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human GnRH receptor expressed in CHO-K1 cells assessed as inhibition of buserelin-induced response preincubated for 20 mins fo... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01076 BindingDB Entry DOI: 10.7270/Q26D5XK9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50548493 (BAY-784) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Tag-lite green-labeled agonist binding to terbium fluorophore-labeled human N-terminal SNAP-tag GnRh receptor expressed in HEK293 cells... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01076 BindingDB Entry DOI: 10.7270/Q26D5XK9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50548473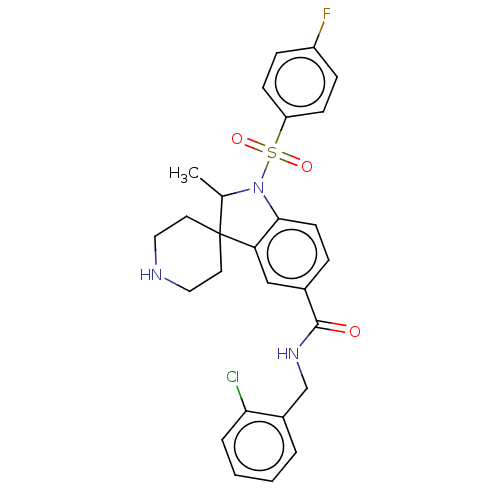 (CHEMBL4740373) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at rat GnRH receptor expressed in CHO-K1 cells assessed as inhibition of LHRH-induced response preincubated for 20 mins followed ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01076 BindingDB Entry DOI: 10.7270/Q26D5XK9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50548473 (CHEMBL4740373) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at rat GnRH receptor expressed in CHO-K1 cells assessed as inhibition of LHRH-induced response preincubated for 20 mins followed ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01076 BindingDB Entry DOI: 10.7270/Q26D5XK9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50548472 (CHEMBL4749790) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human GnRH receptor expressed in CHO-K1 cells assessed as inhibition of buserelin-induced response preincubated for 20 mins fo... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01076 BindingDB Entry DOI: 10.7270/Q26D5XK9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50548472 (CHEMBL4749790) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human GnRH receptor expressed in CHO-K1 cells assessed as inhibition of buserelin-induced response preincubated for 20 mins fo... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01076 BindingDB Entry DOI: 10.7270/Q26D5XK9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50548472 (CHEMBL4749790) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at rat GnRH receptor expressed in CHO-K1 cells assessed as inhibition of LHRH-induced response preincubated for 20 mins followed ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01076 BindingDB Entry DOI: 10.7270/Q26D5XK9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 172 total ) | Next | Last >> |