 Found 194 hits with Last Name = 'lammi' and Initial = 'c'
Found 194 hits with Last Name = 'lammi' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50299702
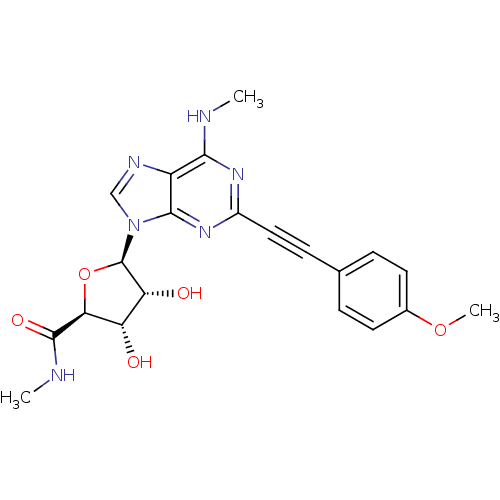
(2-(4-Acetylphenyl)ethynyl-N6-methyl-5'-N-methylcar...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NC)nc(nc12)C#Cc1ccc(OC)cc1 |r| Show InChI InChI=1S/C21H22N6O5/c1-22-18-14-19(26-13(25-18)9-6-11-4-7-12(31-3)8-5-11)27(10-24-14)21-16(29)15(28)17(32-21)20(30)23-2/h4-5,7-8,10,15-17,21,28-29H,1-3H3,(H,23,30)(H,22,25,26)/t15-,16+,17-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino
Curated by ChEMBL
| Assay Description
Displacement of [3H]NECA from human recombinant adenosine A3 receptor expressed in CHO cells |
J Med Chem 52: 7897-900 (2009)
Article DOI: 10.1021/jm900754g
BindingDB Entry DOI: 10.7270/Q2R211G6 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50299697
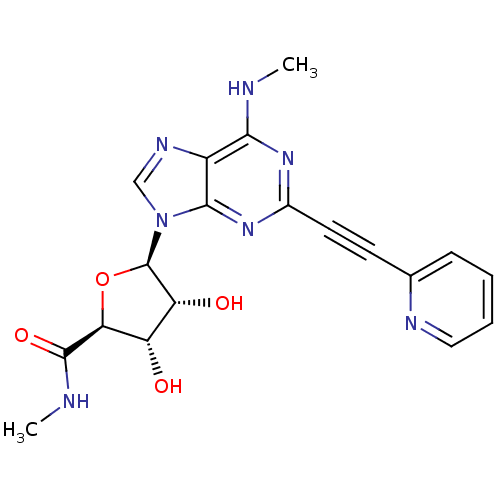
(2-(2-Pyridinyl)ethynyl-N6-methyl-5'-N-methylcarbox...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NC)nc(nc12)C#Cc1ccccn1 |r| Show InChI InChI=1S/C19H19N7O4/c1-20-16-12-17(25-11(24-16)7-6-10-5-3-4-8-22-10)26(9-23-12)19-14(28)13(27)15(30-19)18(29)21-2/h3-5,8-9,13-15,19,27-28H,1-2H3,(H,21,29)(H,20,24,25)/t13-,14+,15-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino
Curated by ChEMBL
| Assay Description
Displacement of [3H]NECA from human recombinant adenosine A3 receptor expressed in CHO cells |
J Med Chem 52: 7897-900 (2009)
Article DOI: 10.1021/jm900754g
BindingDB Entry DOI: 10.7270/Q2R211G6 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50299703

(2-(4-Fluorophenyl)ethynyl-N6-methyl-5'-N-methylcar...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NC)nc(nc12)C#Cc1ccc(F)cc1 |r| Show InChI InChI=1S/C20H19FN6O4/c1-22-17-13-18(26-12(25-17)8-5-10-3-6-11(21)7-4-10)27(9-24-13)20-15(29)14(28)16(31-20)19(30)23-2/h3-4,6-7,9,14-16,20,28-29H,1-2H3,(H,23,30)(H,22,25,26)/t14-,15+,16-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino
Curated by ChEMBL
| Assay Description
Displacement of [3H]NECA from human recombinant adenosine A3 receptor expressed in CHO cells |
J Med Chem 52: 7897-900 (2009)
Article DOI: 10.1021/jm900754g
BindingDB Entry DOI: 10.7270/Q2R211G6 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50299701

(CHEMBL574602 | N6-Methyl-2-phenylethynyl-5'-N-meth...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NC)nc(nc12)C#Cc1ccccc1 |r| Show InChI InChI=1S/C20H20N6O4/c1-21-17-13-18(25-12(24-17)9-8-11-6-4-3-5-7-11)26(10-23-13)20-15(28)14(27)16(30-20)19(29)22-2/h3-7,10,14-16,20,27-28H,1-2H3,(H,22,29)(H,21,24,25)/t14-,15+,16-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino
Curated by ChEMBL
| Assay Description
Displacement of [3H]NECA from human recombinant adenosine A3 receptor expressed in CHO cells |
J Med Chem 52: 7897-900 (2009)
Article DOI: 10.1021/jm900754g
BindingDB Entry DOI: 10.7270/Q2R211G6 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50205729

((2S,3S,4R,5R)-3,4-dihydroxy-5-(6-(methoxyamino)-2-...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NOC)nc(nc12)C#Cc1ccccn1 Show InChI InChI=1S/C19H19N7O5/c1-20-18(29)15-13(27)14(28)19(31-15)26-9-22-12-16(25-30-2)23-11(24-17(12)26)7-6-10-5-3-4-8-21-10/h3-5,8-9,13-15,19,27-28H,1-2H3,(H,20,29)(H,23,24,25)/t13-,14+,15-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino
Curated by ChEMBL
| Assay Description
Displacement of [3H]NECA from human recombinant adenosine A3 receptor expressed in CHO cells |
J Med Chem 52: 7897-900 (2009)
Article DOI: 10.1021/jm900754g
BindingDB Entry DOI: 10.7270/Q2R211G6 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50205722
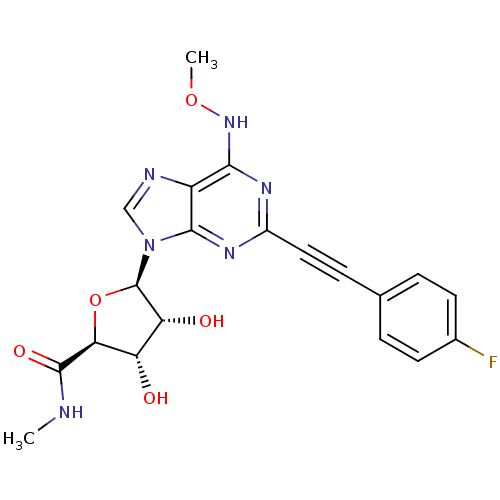
((2S,3S,4R,5R)-5-(2-((4-fluorophenyl)ethynyl)-6-(me...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NOC)nc(nc12)C#Cc1ccc(F)cc1 Show InChI InChI=1S/C20H19FN6O5/c1-22-19(30)16-14(28)15(29)20(32-16)27-9-23-13-17(26-31-2)24-12(25-18(13)27)8-5-10-3-6-11(21)7-4-10/h3-4,6-7,9,14-16,20,28-29H,1-2H3,(H,22,30)(H,24,25,26)/t14-,15+,16-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino
Curated by ChEMBL
| Assay Description
Displacement of [3H]NECA from human recombinant adenosine A3 receptor expressed in CHO cells |
J Med Chem 52: 7897-900 (2009)
Article DOI: 10.1021/jm900754g
BindingDB Entry DOI: 10.7270/Q2R211G6 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50205710
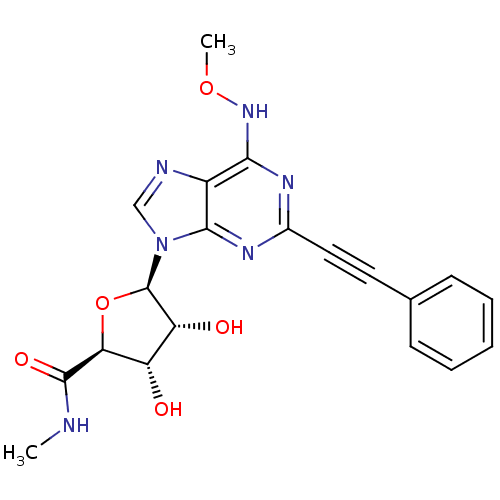
((2S,3S,4R)-3,4-dihydroxy-5-((R)-6-methoxyamino-2-p...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NOC)nc(nc12)C#Cc1ccccc1 Show InChI InChI=1S/C20H20N6O5/c1-21-19(29)16-14(27)15(28)20(31-16)26-10-22-13-17(25-30-2)23-12(24-18(13)26)9-8-11-6-4-3-5-7-11/h3-7,10,14-16,20,27-28H,1-2H3,(H,21,29)(H,23,24,25)/t14-,15+,16-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino
Curated by ChEMBL
| Assay Description
Displacement of [3H]NECA from human recombinant adenosine A3 receptor expressed in CHO cells |
J Med Chem 52: 7897-900 (2009)
Article DOI: 10.1021/jm900754g
BindingDB Entry DOI: 10.7270/Q2R211G6 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50299700

((2S,3S,4R,5R)-3,4-dihydroxy-5-(6-(methoxyamino)-2-...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NOC)nc(nc12)C#Cc1ccc(OC)cc1 |r| Show InChI InChI=1S/C21H22N6O6/c1-22-20(30)17-15(28)16(29)21(33-17)27-10-23-14-18(26-32-3)24-13(25-19(14)27)9-6-11-4-7-12(31-2)8-5-11/h4-5,7-8,10,15-17,21,28-29H,1-3H3,(H,22,30)(H,24,25,26)/t15-,16+,17-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino
Curated by ChEMBL
| Assay Description
Displacement of [3H]NECA from human recombinant adenosine A3 receptor expressed in CHO cells |
J Med Chem 52: 7897-900 (2009)
Article DOI: 10.1021/jm900754g
BindingDB Entry DOI: 10.7270/Q2R211G6 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50299698
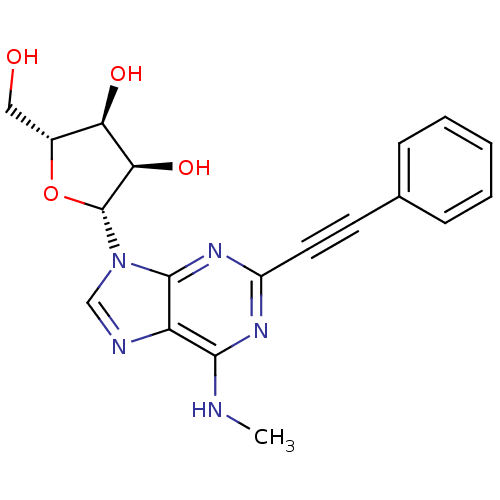
((2R,3S,4R,5R)-2-(hydroxymethyl)-5-(6-(methylamino)...)Show SMILES CNc1nc(nc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O)C#Cc1ccccc1 |r| Show InChI InChI=1S/C19H19N5O4/c1-20-17-14-18(23-13(22-17)8-7-11-5-3-2-4-6-11)24(10-21-14)19-16(27)15(26)12(9-25)28-19/h2-6,10,12,15-16,19,25-27H,9H2,1H3,(H,20,22,23)/t12-,15-,16-,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino
Curated by ChEMBL
| Assay Description
Displacement of [3H]NECA from human recombinant adenosine A3 receptor expressed in CHO cells |
J Med Chem 52: 7897-900 (2009)
Article DOI: 10.1021/jm900754g
BindingDB Entry DOI: 10.7270/Q2R211G6 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50299699
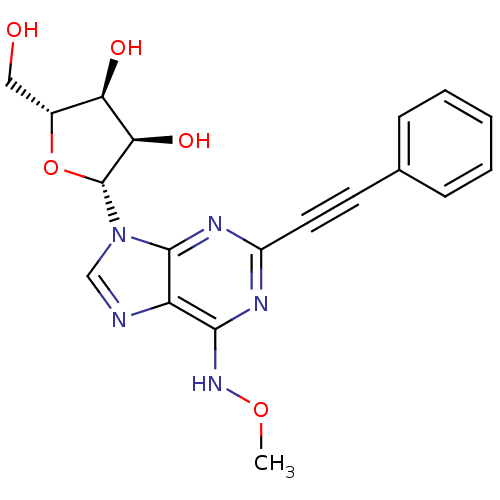
((2R,3S,4R,5R)-2-(hydroxymethyl)-5-(6-(methoxyamino...)Show SMILES CONc1nc(nc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O)C#Cc1ccccc1 |r| Show InChI InChI=1S/C19H19N5O5/c1-28-23-17-14-18(22-13(21-17)8-7-11-5-3-2-4-6-11)24(10-20-14)19-16(27)15(26)12(9-25)29-19/h2-6,10,12,15-16,19,25-27H,9H2,1H3,(H,21,22,23)/t12-,15-,16-,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino
Curated by ChEMBL
| Assay Description
Displacement of [3H]NECA from human recombinant adenosine A3 receptor expressed in CHO cells |
J Med Chem 52: 7897-900 (2009)
Article DOI: 10.1021/jm900754g
BindingDB Entry DOI: 10.7270/Q2R211G6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50591297
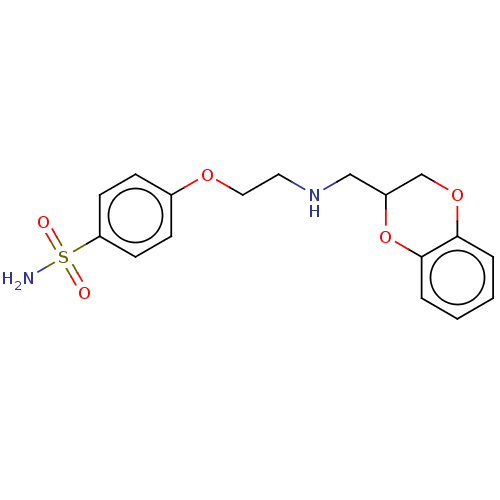
(CHEMBL5171860) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01192
BindingDB Entry DOI: 10.7270/Q2PG1WQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01192
BindingDB Entry DOI: 10.7270/Q2PG1WQ6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50591297

(CHEMBL5171860) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01192
BindingDB Entry DOI: 10.7270/Q2PG1WQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01192
BindingDB Entry DOI: 10.7270/Q2PG1WQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50591309
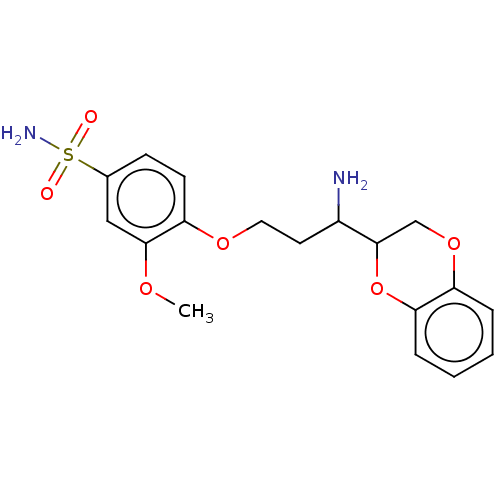
(CHEMBL5193531)Show SMILES COc1cc(ccc1OCCC(N)C1COc2ccccc2O1)S(N)(=O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01192
BindingDB Entry DOI: 10.7270/Q2PG1WQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50591308
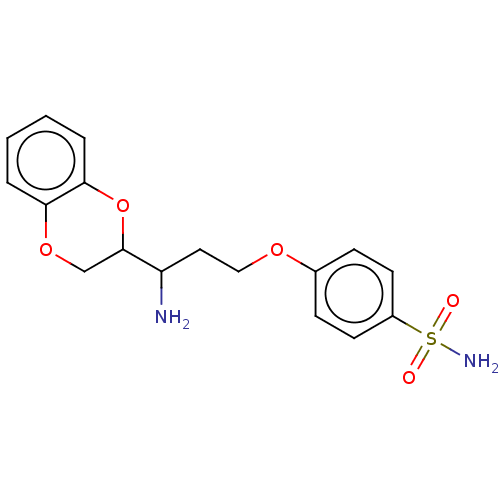
(CHEMBL5177166) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01192
BindingDB Entry DOI: 10.7270/Q2PG1WQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 5B, mitochondrial
(Homo sapiens (Human)) | BDBM50591308

(CHEMBL5177166) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01192
BindingDB Entry DOI: 10.7270/Q2PG1WQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 5B, mitochondrial
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01192
BindingDB Entry DOI: 10.7270/Q2PG1WQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 5A, mitochondrial
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01192
BindingDB Entry DOI: 10.7270/Q2PG1WQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01192
BindingDB Entry DOI: 10.7270/Q2PG1WQ6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 5A, mitochondrial
(Homo sapiens (Human)) | BDBM50591297

(CHEMBL5171860) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01192
BindingDB Entry DOI: 10.7270/Q2PG1WQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 5A, mitochondrial
(Homo sapiens (Human)) | BDBM50591306
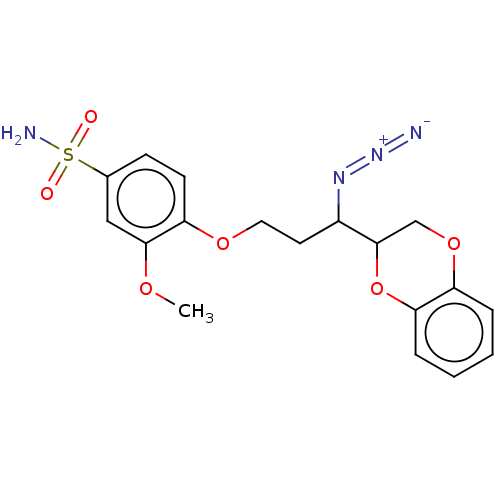
(CHEMBL5206696)Show SMILES COc1cc(ccc1OCCC(N=[N+]=[N-])C1COc2ccccc2O1)S(N)(=O)=O | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01192
BindingDB Entry DOI: 10.7270/Q2PG1WQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50591297

(CHEMBL5171860) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01192
BindingDB Entry DOI: 10.7270/Q2PG1WQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 5B, mitochondrial
(Homo sapiens (Human)) | BDBM50591297

(CHEMBL5171860) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01192
BindingDB Entry DOI: 10.7270/Q2PG1WQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 5A, mitochondrial
(Homo sapiens (Human)) | BDBM50591307
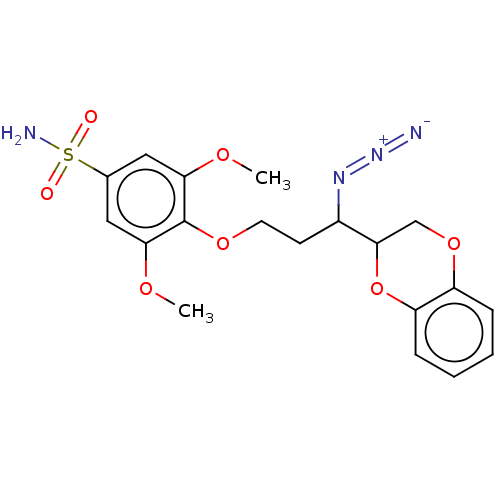
(CHEMBL5198237)Show SMILES COc1cc(cc(OC)c1OCCC(N=[N+]=[N-])C1COc2ccccc2O1)S(N)(=O)=O | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01192
BindingDB Entry DOI: 10.7270/Q2PG1WQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 5A, mitochondrial
(Homo sapiens (Human)) | BDBM50591308

(CHEMBL5177166) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01192
BindingDB Entry DOI: 10.7270/Q2PG1WQ6 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM80642
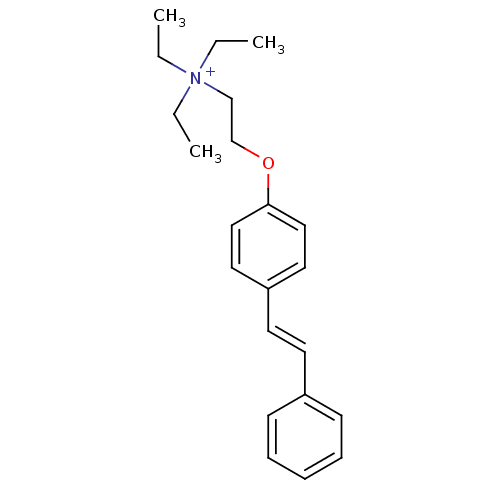
(CHEMBL191491 | MG 624 | MLS002172460 | SMR00125409...)Show InChI InChI=1S/C22H30NO/c1-4-23(5-2,6-3)18-19-24-22-16-14-21(15-17-22)13-12-20-10-8-7-9-11-20/h7-17H,4-6,18-19H2,1-3H3/q+1/b13-12+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 104 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano
Curated by ChEMBL
| Assay Description
Displacement of [125I]-alpha-bungarotoxin from human alpha7 nAChR expressed in human SH-SY5Y cell membranes after 30 mins by gamma counting analysis |
J Med Chem 61: 10531-10544 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01052
BindingDB Entry DOI: 10.7270/Q2PV6P2W |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50591306

(CHEMBL5206696)Show SMILES COc1cc(ccc1OCCC(N=[N+]=[N-])C1COc2ccccc2O1)S(N)(=O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 111 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01192
BindingDB Entry DOI: 10.7270/Q2PG1WQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50591308

(CHEMBL5177166) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 133 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01192
BindingDB Entry DOI: 10.7270/Q2PG1WQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 5B, mitochondrial
(Homo sapiens (Human)) | BDBM50591307

(CHEMBL5198237)Show SMILES COc1cc(cc(OC)c1OCCC(N=[N+]=[N-])C1COc2ccccc2O1)S(N)(=O)=O | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01192
BindingDB Entry DOI: 10.7270/Q2PG1WQ6 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50466608

(CHEMBL4285772)Show SMILES [I-].CC[N+](CC)(CC)CCCCCCCCOc1ccc(\C=C\c2ccccc2)cc1 Show InChI InChI=1S/C28H42NO/c1-4-29(5-2,6-3)24-14-9-7-8-10-15-25-30-28-22-20-27(21-23-28)19-18-26-16-12-11-13-17-26/h11-13,16-23H,4-10,14-15,24-25H2,1-3H3/q+1/b19-18+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 164 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano
Curated by ChEMBL
| Assay Description
Displacement of [125I]-alpha-bungarotoxin from human alpha7 nAChR expressed in human SH-SY5Y cell membranes after 30 mins by gamma counting analysis |
J Med Chem 61: 10531-10544 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01052
BindingDB Entry DOI: 10.7270/Q2PV6P2W |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 5B, mitochondrial
(Homo sapiens (Human)) | BDBM50591305
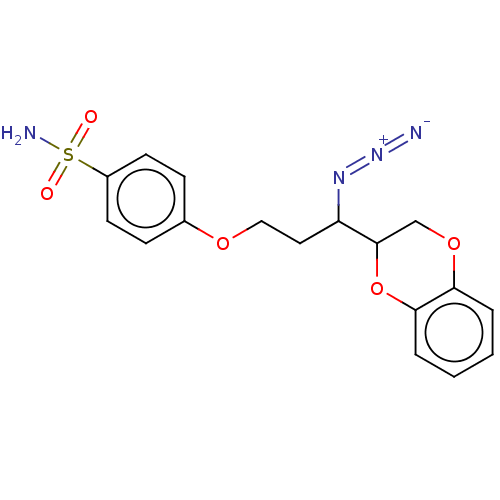
(CHEMBL5191973)Show SMILES NS(=O)(=O)c1ccc(OCCC(N=[N+]=[N-])C2COc3ccccc3O2)cc1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 183 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01192
BindingDB Entry DOI: 10.7270/Q2PG1WQ6 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50466614

(CHEMBL4291079)Show SMILES [I-].CC[N+](CC)(CC)CCCCOc1ccc(\C=C\c2ccccc2)cc1 Show InChI InChI=1S/C24H34NO/c1-4-25(5-2,6-3)20-10-11-21-26-24-18-16-23(17-19-24)15-14-22-12-8-7-9-13-22/h7-9,12-19H,4-6,10-11,20-21H2,1-3H3/q+1/b15-14+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 186 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano
Curated by ChEMBL
| Assay Description
Displacement of [125I]-alpha-bungarotoxin from human alpha7 nAChR expressed in human SH-SY5Y cell membranes after 30 mins by gamma counting analysis |
J Med Chem 61: 10531-10544 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01052
BindingDB Entry DOI: 10.7270/Q2PV6P2W |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50466613

(CHEMBL4287656)Show SMILES [I-].CC[N+](CC)(CC)CCOc1ccc(\C=C\c2cc(OC)cc(OC)c2)cc1 Show InChI InChI=1S/C24H34NO3/c1-6-25(7-2,8-3)15-16-28-22-13-11-20(12-14-22)9-10-21-17-23(26-4)19-24(18-21)27-5/h9-14,17-19H,6-8,15-16H2,1-5H3/q+1/b10-9+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 189 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano
Curated by ChEMBL
| Assay Description
Displacement of [125I]-alpha-bungarotoxin from human alpha7 nAChR expressed in human SH-SY5Y cell membranes after 30 mins by gamma counting analysis |
J Med Chem 61: 10531-10544 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01052
BindingDB Entry DOI: 10.7270/Q2PV6P2W |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 5B, mitochondrial
(Homo sapiens (Human)) | BDBM50591310
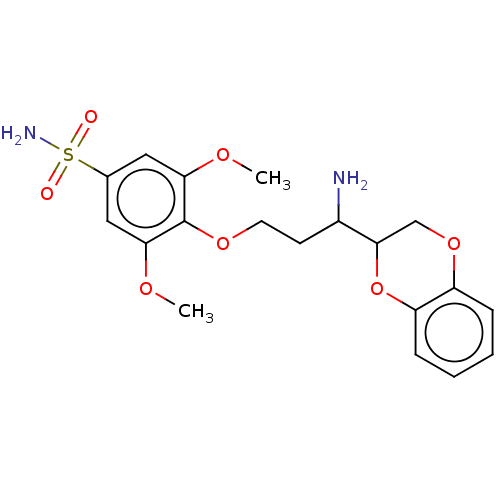
(CHEMBL5205289)Show SMILES COc1cc(cc(OC)c1OCCC(N)C1COc2ccccc2O1)S(N)(=O)=O | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 232 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01192
BindingDB Entry DOI: 10.7270/Q2PG1WQ6 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50591308

(CHEMBL5177166) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 245 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01192
BindingDB Entry DOI: 10.7270/Q2PG1WQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01192
BindingDB Entry DOI: 10.7270/Q2PG1WQ6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50591308

(CHEMBL5177166) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 262 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01192
BindingDB Entry DOI: 10.7270/Q2PG1WQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50591297

(CHEMBL5171860) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 291 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01192
BindingDB Entry DOI: 10.7270/Q2PG1WQ6 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50466606
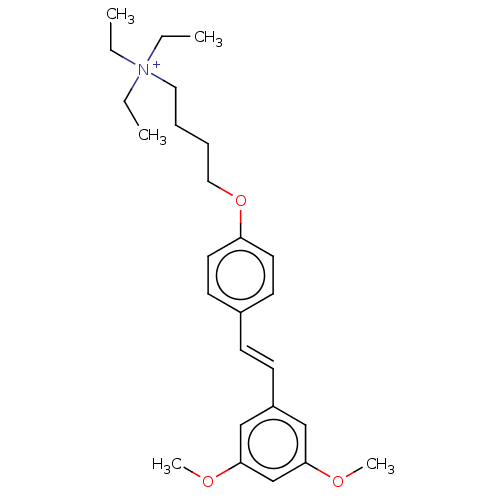
(CHEMBL4280452)Show SMILES [I-].CC[N+](CC)(CC)CCCCOc1ccc(\C=C\c2cc(OC)cc(OC)c2)cc1 Show InChI InChI=1S/C26H38NO3/c1-6-27(7-2,8-3)17-9-10-18-30-24-15-13-22(14-16-24)11-12-23-19-25(28-4)21-26(20-23)29-5/h11-16,19-21H,6-10,17-18H2,1-5H3/q+1/b12-11+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 305 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano
Curated by ChEMBL
| Assay Description
Displacement of [125I]-alpha-bungarotoxin from human alpha7 nAChR expressed in human SH-SY5Y cell membranes after 30 mins by gamma counting analysis |
J Med Chem 61: 10531-10544 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01052
BindingDB Entry DOI: 10.7270/Q2PV6P2W |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50591305

(CHEMBL5191973)Show SMILES NS(=O)(=O)c1ccc(OCCC(N=[N+]=[N-])C2COc3ccccc3O2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 328 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01192
BindingDB Entry DOI: 10.7270/Q2PG1WQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50591306

(CHEMBL5206696)Show SMILES COc1cc(ccc1OCCC(N=[N+]=[N-])C1COc2ccccc2O1)S(N)(=O)=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01192
BindingDB Entry DOI: 10.7270/Q2PG1WQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 5B, mitochondrial
(Homo sapiens (Human)) | BDBM50591306

(CHEMBL5206696)Show SMILES COc1cc(ccc1OCCC(N=[N+]=[N-])C1COc2ccccc2O1)S(N)(=O)=O | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 387 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01192
BindingDB Entry DOI: 10.7270/Q2PG1WQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 5B, mitochondrial
(Homo sapiens (Human)) | BDBM50591309

(CHEMBL5193531)Show SMILES COc1cc(ccc1OCCC(N)C1COc2ccccc2O1)S(N)(=O)=O | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01192
BindingDB Entry DOI: 10.7270/Q2PG1WQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 5A, mitochondrial
(Homo sapiens (Human)) | BDBM50591309

(CHEMBL5193531)Show SMILES COc1cc(ccc1OCCC(N)C1COc2ccccc2O1)S(N)(=O)=O | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 456 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01192
BindingDB Entry DOI: 10.7270/Q2PG1WQ6 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
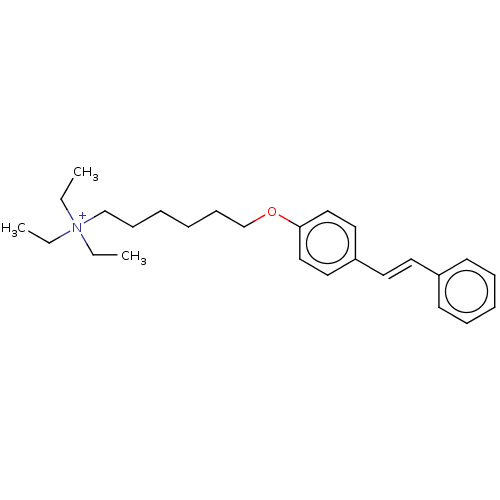
(Homo sapiens (Human)) | BDBM50466607
(CHEMBL4293646)Show SMILES [I-].CC[N+](CC)(CC)CCCCCCOc1ccc(\C=C\c2ccccc2)cc1 Show InChI InChI=1S/C26H38NO/c1-4-27(5-2,6-3)22-12-7-8-13-23-28-26-20-18-25(19-21-26)17-16-24-14-10-9-11-15-24/h9-11,14-21H,4-8,12-13,22-23H2,1-3H3/q+1/b17-16+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 465 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano
Curated by ChEMBL
| Assay Description
Displacement of [125I]-alpha-bungarotoxin from human alpha7 nAChR expressed in human SH-SY5Y cell membranes after 30 mins by gamma counting analysis |
J Med Chem 61: 10531-10544 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01052
BindingDB Entry DOI: 10.7270/Q2PV6P2W |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50591309

(CHEMBL5193531)Show SMILES COc1cc(ccc1OCCC(N)C1COc2ccccc2O1)S(N)(=O)=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 478 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01192
BindingDB Entry DOI: 10.7270/Q2PG1WQ6 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50466603
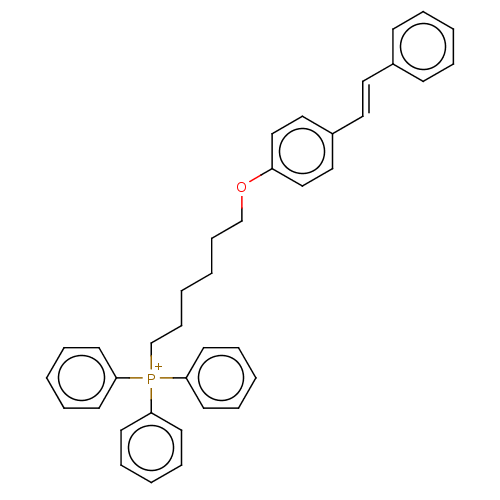
(CHEMBL4277852)Show SMILES [I-].C(CCC[P+](c1ccccc1)(c1ccccc1)c1ccccc1)CCOc1ccc(\C=C\c2ccccc2)cc1 Show InChI InChI=1S/C38H38OP/c1(15-31-39-35-29-27-34(28-30-35)26-25-33-17-7-3-8-18-33)2-16-32-40(36-19-9-4-10-20-36,37-21-11-5-12-22-37)38-23-13-6-14-24-38/h3-14,17-30H,1-2,15-16,31-32H2/q+1/b26-25+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 559 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano
Curated by ChEMBL
| Assay Description
Displacement of [125I]-alpha-bungarotoxin from human alpha7 nAChR expressed in human SH-SY5Y cell membranes after 30 mins by gamma counting analysis |
J Med Chem 61: 10531-10544 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01052
BindingDB Entry DOI: 10.7270/Q2PV6P2W |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50466615
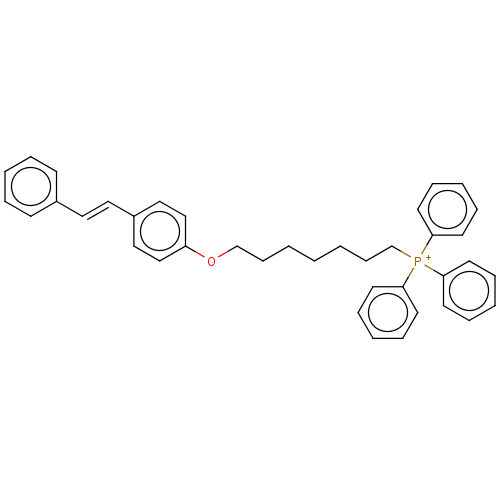
(CHEMBL4291927)Show SMILES [I-].C(CCCOc1ccc(\C=C\c2ccccc2)cc1)CCC[P+](c1ccccc1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C39H40OP/c1(2-16-32-40-36-30-28-35(29-31-36)27-26-34-18-8-4-9-19-34)3-17-33-41(37-20-10-5-11-21-37,38-22-12-6-13-23-38)39-24-14-7-15-25-39/h4-15,18-31H,1-3,16-17,32-33H2/q+1/b27-26+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 617 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano
Curated by ChEMBL
| Assay Description
Displacement of [125I]-alpha-bungarotoxin from human alpha7 nAChR expressed in human SH-SY5Y cell membranes after 30 mins by gamma counting analysis |
J Med Chem 61: 10531-10544 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01052
BindingDB Entry DOI: 10.7270/Q2PV6P2W |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 5A, mitochondrial
(Homo sapiens (Human)) | BDBM50591305

(CHEMBL5191973)Show SMILES NS(=O)(=O)c1ccc(OCCC(N=[N+]=[N-])C2COc3ccccc3O2)cc1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 659 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01192
BindingDB Entry DOI: 10.7270/Q2PG1WQ6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data